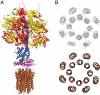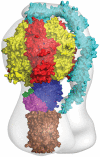Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria - PubMed (original) (raw)
Bioenergetic cost of making an adenosine triphosphate molecule in animal mitochondria
Ian N Watt et al. Proc Natl Acad Sci U S A. 2010.
Abstract
The catalytic domain of the F-ATPase in mitochondria protrudes into the matrix of the organelle, and is attached to the membrane domain by central and peripheral stalks. Energy for the synthesis of ATP from ADP and phosphate is provided by the transmembrane proton-motive-force across the inner membrane, generated by respiration. The proton-motive force is coupled mechanically to ATP synthesis by the rotation at about 100 times per second of the central stalk and an attached ring of c-subunits in the membrane domain. Each c-subunit carries a glutamate exposed around the midpoint of the membrane on the external surface of the ring. The rotation is generated by protonation and deprotonation successively of each glutamate. Each 360° rotation produces three ATP molecules, and requires the translocation of one proton per glutamate by each c-subunit in the ring. In fungi, eubacteria, and plant chloroplasts, ring sizes of c(10)-c(15) subunits have been observed, implying that these enzymes need 3.3-5 protons to make each ATP, but until now no higher eukaryote has been examined. As shown here in the structure of the bovine F(1)-c-ring complex, the c-ring has eight c-subunits. As the sequences of c-subunits are identical throughout almost all vertebrates and are highly conserved in invertebrates, their F-ATPases probably contain c(8)-rings also. Therefore, in about 50,000 vertebrate species, and probably in many or all of the two million invertebrate species, 2.7 protons are required by the F-ATPase to make each ATP molecule.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
The structure of the bovine F1-c-ring complex. Part A. The subunits in the F1-catalytic domain (above) and the c-ring (below) are shown in ribbon representation. The membrane extrinsic region consists of the spherical catalytic domain, made of three α- and three β-subunits (red and yellow, respectively), the central stalk (subunits γ, δ, and ϵ, blue, purple, and green, respectively), The central stalk and the c-ring (brown) together constitute the rotor. Each of the C-terminal α-helices in the c-ring has a glutamate at residue 58, at the midpoint of the lipid bilayer. Part B. Cross-section of the c-ring taken at the midpoint of the α-helices. Above is shown the electron density with the eight N-terminal α-helices in the inner ring, and the eight C-terminal α-helices in the outer ring, and below the structural interpretation of the density.
Fig. 2.
The mosaic structure of the F-ATPase from bovine mitochondria. The components of the structure were docked into a structure of the intact enzyme determined by electron cryomicroscopy (pale gray outline). The F1-domain and the c-ring are taken from the present work. In addition many, structures of the isolated F1-domain have been determined, the most accurate at 1.9 Å resolution. The peripheral stalk (cyan; made of single copies of subunits OSCP, b, d, and F6) penetrates into the membrane domain and links subunit a to the external surface of the catalytic domain. The structure of the peripheral stalk is derived from a structure of bovine F1-ATPase with most of the peripheral stalk attached to it, augmented by information from a structure of an overlapping fragment of the peripheral stalk. The foot of the central stalk makes extensive contacts with the bovine c-ring (this work). The empty gray region on the right represents the part of the structure in the membrane domain of the enzyme where detailed structural information is lacking for subunit a (ATPase-6), which sits close to the surface of the c-ring and provides a transmembrane path for protons. Also, it contains the membrane intrinsic region of subunit b (two transmembrane α-helices), and subunits A6L, e, f, and g (each with a single transmembrane α-helix), which are not involved directly in the synthesis of ATP. It is likely that, as in the 16 Å resolution structure of the V-ATPase from Thermus thermophilus, the gray area in the membrane domain will contain an annular belt of detergent.
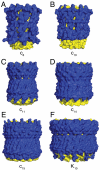
Fig. 3.
External surfaces of rotor rings from F- and V-ATPases. The rings are shown in solid representation. The N- and C-terminal α-helices are yellow and blue, respectively. Parts A_–_E, c-rings from F-ATPases from bovine and yeast mitochondria, from Ilyobacter tartaricus, from Spinacea oleracea, and from Spirulina platensis. Part F, the K-ring from the V-ATPase from Enterococcus hirae.
Fig. 4.
Sequences of c-subunits from F-ATP synthase in vertebrates. The sequences have been aligned from the following species with their names indicated on the left, and the animal class to which the species belongs is shown on the right: HOMSA, Homo sapiens (human); PONAB, Pongo albelii (Sumatran orangutan); BOSTA, Bos taurus (cattle); OVIAR; Ovis aries (sheep); CANFA, Canis lupus familiaris (dog); SUSSC, Sus scrofa (pig); EQUCA, Equus caballus (horse); AILME, Ailuropoda melanoleuca (giant panda); ORYCU, Oryctolagus cuniculus (rabbit); RATTU, Rattus norvegicus (Norwegian rat); MICMU, Microcebus murinus (mouse lemur); OTOGA, Otolemur garnettii (small eared galago); VICPA, Vicugna pacos (alpaca); MONDE, Mondelphis domestica (gray short tailed opposum); MACEU, Macropus eugenii (wallaby); ORNAN; Ornithorhynchus anatinus (duckbill platypus); TURTR, Tursiops truncates (bottle nosed dolphin); ANOCA, Anolis carolinesis (green anole lizard); GALGA, Gallus gallus (chicken); MELGA, Meleagris gallopavo (turkey); TAEGU, Taenio guttat (zebra finch); XENLA, Xenopus laevis (clawed toad); DANRE, Danio rerio (zebrafish); TETNG, Tetraodon nigroviridis (green pufferfish); TAKRU, Takifugu rubripes (fugu pufferfish); ICTPU, Ictalurus punctatus (channel catfish); ANOFI, Anoplopoma fimbria (sablefish); ESOLU, Esox lucius (northern pike); OSSMO, Osmerus mordax (rainbow smelt); SALSA, Salmo salar (Atlantic salmon); CYPCA, Cyprinus carpio (common carp); and PAROL, Paralichthys olivaceus (Japanese flounder). The green boxes indicate alanines 13, 19, and 23 that are required for the formation of the c8-ring. The purple box and blue boxes show, respectively, the positions of the lysine residue that is known to be trimethylated in the human, bovine, ovine, porcine, and rabbit enzymes and of the glutamate residue that is involved in proton translocation through the inner membranes of mitochondria
Fig. 5.
Sequences of c-subunits from F-ATP synthase in invertebrates compared with the human sequence. The sequences have been aligned from the following species with their names indicated on the left, and the phylum to which the species belongs is shown on the right: BRABE, Branchiostoma belcheri (amphioxus or Japanese lancelet); PENJP, Peneus japonica (Kuruma prawn); DROME, Drosophila melanogaster (fruit fly); MANSE, Manduca sexta (tobacco hawkmoth); AEDAE, Aedes aegypti (yellow fever mosquito); ANOGA, Anopheles gambiae (African malarial mosquito); RHISA, Rhicephalus sanguineus (brown dog tick); GLOMM, Glossina morsitans (savannah tsetse fly); APIME, Apis mellifera (honey bee); TRICA, Tribolium castaneum (red flour beetle); CAEEL, Caenorhabditis elegans; LOTGA, Lottia gigantea (sea snail); HYDEI, Hydroides elegans (tube-worm; the C-terminal sequence continues for 17 more residues); HELRO, Helobdella robusta (leech); STRPU, Stronglycentrotus purpuratus (purple sea urchin); SCHMA, Schistosoma mansoni (parasitic worm); NEMVE, Nematostella vectensis (starlet sea anenome); CARBA, Carukia barnesi (Irukandji jellyfish); TRIAD, Trichoplax adhaerans (presponge, simplest nonparasitic invertebrate); SUBDO, Suberites domuncula (sponge); and OSSCA, Oscarella carmela (sponge). Amino acid substitutions are shown in red. For the explanation of the colored boxes, see the legend to Fig. 4.
Comment in
- ATP synthase: from sequence to ring size to the P/O ratio.
Ferguson SJ. Ferguson SJ. Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):16755-6. doi: 10.1073/pnas.1012260107. Epub 2010 Sep 21. Proc Natl Acad Sci U S A. 2010. PMID: 20858734 Free PMC article. No abstract available.
Similar articles
- Coupling H+ transport and ATP synthesis in F1F0-ATP synthases: glimpses of interacting parts in a dynamic molecular machine.
Fillingame RH. Fillingame RH. J Exp Biol. 1997 Jan;200(Pt 2):217-24. doi: 10.1242/jeb.200.2.217. J Exp Biol. 1997. PMID: 9050229 Review. - Conservation of complete trimethylation of lysine-43 in the rotor ring of c-subunits of metazoan adenosine triphosphate (ATP) synthases.
Walpole TB, Palmer DN, Jiang H, Ding S, Fearnley IM, Walker JE. Walpole TB, et al. Mol Cell Proteomics. 2015 Apr;14(4):828-40. doi: 10.1074/mcp.M114.047456. Epub 2015 Jan 21. Mol Cell Proteomics. 2015. PMID: 25608518 Free PMC article. - The structure of the central stalk in bovine F(1)-ATPase at 2.4 A resolution.
Gibbons C, Montgomery MG, Leslie AG, Walker JE. Gibbons C, et al. Nat Struct Biol. 2000 Nov;7(11):1055-61. doi: 10.1038/80981. Nat Struct Biol. 2000. PMID: 11062563 - Horizontal membrane-intrinsic α-helices in the stator a-subunit of an F-type ATP synthase.
Allegretti M, Klusch N, Mills DJ, Vonck J, Kühlbrandt W, Davies KM. Allegretti M, et al. Nature. 2015 May 14;521(7551):237-40. doi: 10.1038/nature14185. Epub 2015 Feb 23. Nature. 2015. PMID: 25707805 - Recent developments on structural and functional aspects of the F1 sector of H+-linked ATPases.
Vignais PV, Satre M. Vignais PV, et al. Mol Cell Biochem. 1984;60(1):33-71. doi: 10.1007/BF00226299. Mol Cell Biochem. 1984. PMID: 6231469 Review.
Cited by
- Signaling Pathways Concerning Mitochondrial Dysfunction: Implications in Neurodegeneration and Possible Molecular Targets.
Sharma Y, Gupta JK, Babu MA, Singh S, Sindhu RK. Sharma Y, et al. J Mol Neurosci. 2024 Oct 28;74(4):101. doi: 10.1007/s12031-024-02269-5. J Mol Neurosci. 2024. PMID: 39466510 Review. - The role of mitochondria in tumor metastasis and advances in mitochondria-targeted cancer therapy.
Chen F, Xue Y, Zhang W, Zhou H, Zhou Z, Chen T, YinWang E, Li H, Ye Z, Gao J, Wang S. Chen F, et al. Cancer Metastasis Rev. 2024 Dec;43(4):1419-1443. doi: 10.1007/s10555-024-10211-9. Epub 2024 Sep 23. Cancer Metastasis Rev. 2024. PMID: 39307891 Free PMC article. Review. - A self-reinforcing cycle hypothesis in heart failure pathogenesis.
Fernandez-Patron C, Lopaschuk GD, Hardy E. Fernandez-Patron C, et al. Nat Cardiovasc Res. 2024 Jun;3(6):627-636. doi: 10.1038/s44161-024-00480-6. Epub 2024 Jun 3. Nat Cardiovasc Res. 2024. PMID: 39196226 Review. - Cobra Venom Cytotoxins as a Tool for Probing Mechanisms of Mitochondrial Energetics and Understanding Mitochondrial Membrane Structure.
Gasanoff ES, Dagda RK. Gasanoff ES, et al. Toxins (Basel). 2024 Jun 25;16(7):287. doi: 10.3390/toxins16070287. Toxins (Basel). 2024. PMID: 39057927 Free PMC article. Review. - Mitochondrial complex-1 as a therapeutic target for cardiac diseases.
Rai NK, Venugopal H, Rajesh R, Ancha P, Venkatesh S. Rai NK, et al. Mol Cell Biochem. 2024 Jul 20. doi: 10.1007/s11010-024-05074-1. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39033212 Review.
References
- Walker JE. ATP synthesis by rotary catalysis (Nobel lecture) Angewandte Chemie International Edition. 1998;37:5000–5011. - PubMed
- von Ballmoos C, Wiedenmann A, Dimroth P. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem. 2009;78:649–672. - PubMed
- Junge W, Sielaff H, Engelbrecht S. Torque generation and elastic power transmission in the rotary FOF1-ATPase. Nature. 2009;459:364–370. - PubMed
- von Ballmoos C, Cook GM, Dimroth P. Unique rotary ATP synthase and its biological diversity. Annu Rev Biophys. 2008;37:43–64. - PubMed
- Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials