EGCG functions through estrogen receptor-mediated activation of ADAM10 in the promotion of non-amyloidogenic processing of APP - PubMed (original) (raw)
EGCG functions through estrogen receptor-mediated activation of ADAM10 in the promotion of non-amyloidogenic processing of APP
Jamie Winderbaum Fernandez et al. FEBS Lett. 2010.
Abstract
Estrogen depletion following menopause has been correlated with an increased risk of developing Alzheimer's disease (AD). We previously explored the beneficial effect of (-)-epigallocatechin-3-gallate (EGCG) on AD mice and found increased non-amyloidogenic processing of amyloid precursor protein (APP) through the α-secretase a disintegrin and metallopeptidase domain 10 (ADAM10). Our results in this study suggest that EGCG-mediated enhancement of non-amyloidogenic processing of APP is mediated by the maturation of ADAM10 via an estrogen receptor-α (ERα)/phosphoinositide 3-kinase/Ak-transforming dependent mechanism, independent of furin-mediated ADAM10 activation. These data support prior assertions that central selective ER modulation could be a therapeutic target for AD and support the use of EGCG as a well-tolerated alternative to estrogen therapy in the prophylaxis and treatment of this disease.
Copyright © 2010 Federation of European Biochemical Societies. All rights reserved.
Figures
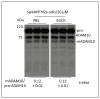
Figure 1. Estrogen receptor (ERα) inhibition mitigates EGCG-induced ADAM10 activation and non-amyloidogenic APP processing in SweAPP N2a cells
SweAPP N2a cells (murine neuroblastoma cells overexpressing Swedish mutant 695aa isoform of APP) were treated with EGCG at 20 μM in the presence of estrogen inhibitor (MPP, an antagonist at ERα receptor displaying > 200-fold selectivity for ERα over ERβ) or a control compound lacking estrogen receptor modulation properties at various doses as indicated for 12 hours. Aβ1-40, 42 peptides were analyzed in conditioned media from these cells by ELISA. Data are represented as Aβ1-40, 42 (pg) in total cellular protein (mg) secreted 12 hours after co-treatment as indicated below the figure. Cell lysates were prepared and subjected to western analysis of ADAM10 maturation. Densitometric analysis shows the ratio of active mature (mADAM10) to proform (pro-ADAM10) as indicated below the figure. One-way ANOVA followed by post-hoc comparison revealed significant differences between MPP doses (**P <0.005 with n = 3 for each condition), but not control inhibitor (_P_ > 0.05), for Aβ generation and ratio of mADAM10 to pro-ADAM10.
Figure 2. EGCG failed to directly promote ADAM10 activation in broken SweAPP N2a cell preparations
Cell lysates from untreated SweAPP N2a cells and subsequently treated these lysates with EGCG (10 μM) or PBS. One hour later, these cell lysates were subjected to western analysis of ADAM10. Densitometric analysis shows the ratio of mADAM10 to pro-ADAM10 as indicated below the figure. One-way ANOVA followed by post-hoc comparison revealed no significant differences between the treated conditions (P >0.05 with n = 4 for each condition) for the ratio of mADAM10 to pro-ADAM10.
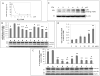
Figure 3. PI3K/Akt signaling is involved in EGCG-mediated ADAM10 activation and promotion of non-amyloidogenic processing of APP
Various treatment conditions in SweAPP N2a cells are denoted as a-j and correspond to the following: a. no treatment, b. EGCG, c. wortmannin (WM) 200 nM + EGCG, d. WM 400 nM + EGCG, e. WM 10 μM + EGCG, f. WM 400nM, g. LY294002 10μM + EGCG, h. LY294002 50 μM+ EGCG, i. LY294002 100 μM + EGCG, j. LY294002 50 μM. EGCG was used at a concentration of 20 μM for all conditions unless otherwise indicated. (A) SweAPP N2a cells were treated with EGCG and sAPPα release was quantitated by ELISA after varying treatment concentrations of PI3K inhibitor (WM). (B) pro-ADAM10 and mADAM10 following treatment with the PI3K inhibitors, WM and LY294002 were analyzed in cell lysates from SweAPP N2a cells by western blot. Densitometry analysis results are represented as band density ratio means ± SEM (n = 3), * P<0.05, ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment. (C, D) SweAPP N2a cell lysates were collected following treatment with various concentrations of EGCG as indicated and analyzed by western analysis for the p85 binding motif of PI3K. Densitometry analysis results are represented as band density ratio of p85 to β-actin (± SEM, n = 3, ** P<0.01). (E) total Akt and phospho-Akt were assessed as percent of associated control following treatment with the PI3K inhibitors and were analyzed in cell lysates from SweAPP N2a cells by western blot. Densitometry analysis results are represented as band density means ± SEM (n = 3), ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment.
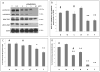
Figure 4. Akt inhibition by triciribine hydrate (TCN) reduces ADAM10 activation, total Akt, and phosphorylated Akt, in the presence of EGCG
Various treatment conditions in SweAPP N2a cells are denoted as a-f and correspond to the following: a. no treatment, b. EGCG, c. TCN 5μM, d. TCN 1 μM + EGCG, e. TCN 5 μM + EGCG, f. TCN 50 μM + EGCG. EGCG was used at a concentration of 20 μM for all conditions unless otherwise indicated. (A) SweAPP N2a cells were treated with varying concentrations of Akt inhibitor (TCN) in the presence and absence of EGCG. Cell lysates were prepared and subjected to western analysis of ADAM10 maturation, total Akt, phospho-Akt (Ser473), and β-actin (internal control). (B) Densitometric analysis shows the ratio of active mature (mADAM10) to proform (pro-ADAM10) as indicated below the figure. One-way ANOVA revealed significant differences between TCN treated and control cells at concentrations of 5 and 10 μM (**P<0.01 with n = 3 for each condition) in the presence of EGCG. # represents the protein of interest compared to control without EGCG treatment. (C, D) Densitometric analysis was performed on total Akt and phospho-Akt (Ser473) and represented as percent of associated control (SweAPP N2a +/- EGCG) following treatment with TCN ± SEM (n = 3), * P<0.05 and ** P<0.01 of protein of interest compared to EGCG control, # represents the protein of interest compared to control without EGCG treatment. Significant differences between treated and control were present at concentrations of 5 and 10 μM for total Akt in the presence of EGCG only, while phospo-Akt was significantly reduced for all TCN concentrations in the presence and absence of EGCG.
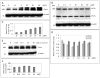
Figure 5. EGCG enhances ADAM10 activating enzyme furin independent of PI3K activation in SweAPP N2a cells
(A, B) Expression of furin and PC7 was analyzed in lysates from SweAPP N2a cells treated with EGCG at concentrations indicated for 4 hours by western blot. (C, D) Densitometric analysis reveals the band density ratio of furin or PC7 isoforms to β-actin (internal reference control). One-way ANOVA followed by post-hoc comparison revealed significant differences (P < 0.01, n=3, data presented as ±SEM) when comparing each concentration of EGCG and respective furin to actin ratio either to control (PBS) or vs. other EGCG dose. Interestingly EGCG treatments did not significantly affect PC7 isoforms expression (B, D). (E) Expression of furin was analyzed in lysates from SweAPP N2a cells treated with EGCG (20μM) in the presence of PI3K inhibitor (wortmannin) at concentrations indicated for 4 hours by western blot. (F) Densitometric analysis reveals the band density ratio of furin to β-actin (internal reference control). One-way ANOVA followed by post-hoc comparison revealed no significant differences (n=3, data presented as ±SEM) when comparing each concentration of PI3K inhibitor and respective furin to β-actin ratio.
Figure 6. Working model of effects of EGCG on APP processing
In this model, EGCG activates membrane associated estrogen receptors in a ligand dependent, non-genomic, manner setting in motion receptor tyrosine phosphorylation of the p85 regulatory subunit of PI3K. Subsequently PIP2 is converted to PIP3, which, in turn, activates Akt to negatively regulate GSK3 and numerous other downstream effectors required for cellular growth and survival. In our model, Akt may also act directly on APP by phosphorylating C-terminal tyrosine sites or indirectly through the adaptor protein Shc to effect APP phosphorylation.[74] Substrate modifications such as phosphorylation and/or association with adaptor proteins may enhance the binding capacity and substrate-mediated activation of ADAM10 directly[71] or indirectly through autoactivation[51] and enhanced expression of furin, also observed after EGCG treatment.
Similar articles
- Octyl gallate markedly promotes anti-amyloidogenic processing of APP through estrogen receptor-mediated ADAM10 activation.
Zhang SQ, Sawmiller D, Li S, Rezai-Zadeh K, Hou H, Zhou S, Shytle D, Giunta B, Fernandez F, Mori T, Tan J. Zhang SQ, et al. PLoS One. 2013 Aug 15;8(8):e71913. doi: 10.1371/journal.pone.0071913. eCollection 2013. PLoS One. 2013. PMID: 23977176 Free PMC article. - ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein.
Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, Zeng J, Mori T, Arendash GW, Shytle D, Town T, Tan J. Obregon DF, et al. J Biol Chem. 2006 Jun 16;281(24):16419-27. doi: 10.1074/jbc.M600617200. Epub 2006 Apr 19. J Biol Chem. 2006. PMID: 16624814 - ADAM10 in Alzheimer's disease: Pharmacological modulation by natural compounds and its role as a peripheral marker.
Manzine PR, Ettcheto M, Cano A, Busquets O, Marcello E, Pelucchi S, Di Luca M, Endres K, Olloquequi J, Camins A, Cominetti MR. Manzine PR, et al. Biomed Pharmacother. 2019 May;113:108661. doi: 10.1016/j.biopha.2019.108661. Epub 2019 Mar 2. Biomed Pharmacother. 2019. PMID: 30836275 Review. - Alpha-lipoic acid alleviates cognitive deficits in transgenic APP23/PS45 mice through a mitophagy-mediated increase in ADAM10 α-secretase cleavage of APP.
Zhang J, Jiang Y, Dong X, Meng Z, Ji L, Kang Y, Liu M, Zhou W, Song W. Zhang J, et al. Alzheimers Res Ther. 2024 Jul 19;16(1):160. doi: 10.1186/s13195-024-01527-3. Alzheimers Res Ther. 2024. PMID: 39030577 Free PMC article. - Activation of α-secretase cleavage.
Postina R. Postina R. J Neurochem. 2012 Jan;120 Suppl 1:46-54. doi: 10.1111/j.1471-4159.2011.07459.x. Epub 2011 Nov 28. J Neurochem. 2012. PMID: 21883223 Review.
Cited by
- Flavonoids as an Intervention for Alzheimer's Disease: Progress and Hurdles Towards Defining a Mechanism of Action.
Hole KL, Williams RJ. Hole KL, et al. Brain Plast. 2021 Feb 9;6(2):167-192. doi: 10.3233/BPL-200098. Brain Plast. 2021. PMID: 33782649 Free PMC article. Review. - Amyloid-beta Alzheimer targets - protein processing, lipid rafts, and amyloid-beta pores.
Arbor SC, LaFontaine M, Cumbay M. Arbor SC, et al. Yale J Biol Med. 2016 Mar 24;89(1):5-21. eCollection 2016 Mar. Yale J Biol Med. 2016. PMID: 27505013 Free PMC article. Review. - Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons.
Patil SP, Tran N, Geekiyanage H, Liu L, Chan C. Patil SP, et al. Neurosci Lett. 2013 Oct 25;554:121-5. doi: 10.1016/j.neulet.2013.09.008. Epub 2013 Sep 11. Neurosci Lett. 2013. PMID: 24035895 Free PMC article. - Cosmosiin Increases ADAM10 Expression via Mechanisms Involving 5'UTR and PI3K Signaling.
Min Z, Tang Y, Hu XT, Zhu BL, Ma YL, Zha JS, Deng XJ, Yan Z, Chen GJ. Min Z, et al. Front Mol Neurosci. 2018 Jun 11;11:198. doi: 10.3389/fnmol.2018.00198. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29942252 Free PMC article. - Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects.
Vauzour D. Vauzour D. Oxid Med Cell Longev. 2012;2012:914273. doi: 10.1155/2012/914273. Epub 2012 Jun 3. Oxid Med Cell Longev. 2012. PMID: 22701758 Free PMC article. Review.
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. - PubMed
- Fahrenholz F. Alpha-secretase as a therapeutic target. Curr Alzheimer Res. 2007;4:412–7. - PubMed
- Fahrenholz F, Tippmann F, Endres K. Retinoids as a perspective in treatment of Alzheimer's disease. Neurodegener Dis. 7:190–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases