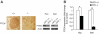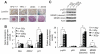Deletion of Pten in pancreatic ß-cells protects against deficient ß-cell mass and function in mouse models of type 2 diabetes - PubMed (original) (raw)
. 2010 Dec;59(12):3117-26.
doi: 10.2337/db09-1805. Epub 2010 Sep 17.
Affiliations
- PMID: 20852026
- PMCID: PMC2992773
- DOI: 10.2337/db09-1805
Deletion of Pten in pancreatic ß-cells protects against deficient ß-cell mass and function in mouse models of type 2 diabetes
Linyuan Wang et al. Diabetes. 2010 Dec.
Abstract
Objective: Type 2 diabetes is characterized by diminished pancreatic β-cell mass and function. Insulin signaling within the β-cells has been shown to play a critical role in maintaining the essential function of the β-cells. Under basal conditions, enhanced insulin-PI3K signaling via deletion of phosphatase with tensin homology (PTEN), a negative regulator of this pathway, leads to increased β-cell mass and function. In this study, we investigated the effects of prolonged β-cell-specific PTEN deletion in models of type 2 diabetes.
Research design and methods: Two models of type 2 diabetes were employed: a high-fat diet (HFD) model and a db/db model that harbors a global leptin-signaling defect. A Cre-loxP system driven by the rat insulin promoter (RIP) was employed to obtain mice with β-cell-specific PTEN deletion (RIPcre(+) Pten(fl/fl)).
Results: PTEN expression in islets was upregulated in both models of type 2 diabetes. RIPcre(+) Pten(fl/fl) mice were completely protected against diabetes in both models of type 2 diabetes. The islets of RIPcre(+) Pten(fl/fl) mice already exhibited increased β-cell mass under basal conditions, and there was no further increase under diabetic conditions. Their β-cell function and islet PI3K signaling remained intact, in contrast to HFD-fed wild-type and db/db islets that exhibited diminished β-cell function and attenuated PI3K signaling. These protective effects in β-cells occurred in the absence of compromised response to DNA-damaging stimuli.
Conclusions: PTEN exerts a critical negative effect on both β-cell mass and function. Thus PTEN inhibition in β-cells can be a novel therapeutic intervention to prevent the decline of β-cell mass and function in type 2 diabetes.
Figures
FIG. 1.
Islet PTEN upregulation with concomitant attenuation of PI3K signaling in models of type 2 diabetes. A and B: PTEN transcript levels by quantitative PCR (A) and immunohistochemical staining (B) of chow- and HFD-fed (after 7 months on HFD); wild-type (+/+) and db/db islets (7 months of age) (n = 3). C: Western blot (left panel) and quantification (right panel) of PTEN, p-Akt (Ser473), total Akt, p-mTOR (Ser2448), total mTOR, p-FoxO-1 (Ser253) and total FoxO-1 in chow- and HFD-fed (7 months on HFD); wild-type and db/db islets (7 months of age) (n = 3). *P < 0.05; **P < 0.005. Scale bar, 50 μm. The results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 2.
PTEN deletion in RIP_cre_+ Pten_fl/fl islets. A: Immunohistochemical staining of PTEN in RIP_cre+ Pten+/+ (+/+) and RIP_cre_+ Pten_fl/fl (−/−) pancreas sections. B: Western blot (left panel) and quantification (right panel) of PTEN expression in RIP_cre+ _Pten_fl/fl islets and hypothalamus (Hyp) (n = 3). *P < 0.05; **P < 0.005. Scale bar, 50 μm. The results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 3.
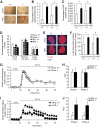
RIP_cre_+ Pten_fl/fl mice showed maintained glucose metabolism and in vivo glucose stimulated insulin secretion after prolonged HFD while demonstrating drastic weight gain. A: Weight of RIP_cre+ Pten+/+ (+/+) and RIP_cre_+ Pten_fl/fl (−/−) mice at the start of HFD (2 months of age) and after HFD (9 months of age) with chow-fed RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice at the same time points. B: Fasting blood glucose of RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice fed either chow or HFD for 7 months (n >7). C: Glucose tolerance tests of RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice after 7 months of either chow or HFD feeding (n >7). D: in vivo glucose stimulated insulin secretions of RIP_cre+ Pten+/+ and RIP_cre_+ _Pten_fl/fl mice after 7 months of either chow or HFD feeding (n >3). *P < 0.05. The results are presented as mean ± SE.
FIG. 4.
RIP_cre_+ Pten_fl/fl mice maintained high islet mass and β-cell size with protection against HFD-induced β-cell dysfunction. A and B: Insulin staining (A) and quantification (B) of pancreas sections of RIP_cre+ Pten+/+ (+/+) and RIP_cre_+ Pten_fl/fl (−/−) mice fed either chow or HFD (n = 3), Scale bar, 500 μm. C: Percentage of Ki67 positive cells in islets from RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice fed either chow or HFD (n = 3). D: proportion of small (<10 cells), medium (10–200 cells) and large (>200 cells) islet in pancreas from RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice fed either chow or HFD (n = 3). E and F: Immunofluorescent staining of insulin/DAPI (E) and quantification of β-cell size (F) of pancreas from RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl mice fed either chow or HFD (n = 3), Scale bar, 50 μm. G and H: Insulin secretion per 60 islets during perifusion analysis (G) and quantification of area under the curve (AUC) (H) of chow-fed RIP_cre+ Pten+/+ and RIP_cre_+ Pten_fl/fl islets (n = 3). I and J: Insulin secretion per 60 islets during perifusion analysis (I) and quantification of area under the curve (J) of HFD-fed RIP_cre+ Pten+/+ and RIP_cre_+ _Pten_fl/fl islets (n = 3). *P < 0.05. Results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 5.
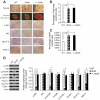
RIP_cre_+ Pten_fl/fl mice maintained islet PI3K signaling after prolonged HFD. A: Immunofluorescent staining of insulin/glucagon, and immunohistochemical staining of p-Akt (Ser473), total Akt, GLUT2, and PDX-1 in chow-fed, HFD-fed RIP_cre+ Pten+/+ (+/+) and HFD-fed RIP_cre_+ Pten_fl/fl (−/−) mice. B: Western blot (left panel) and quantification (right panel) of p-Akt (Ser473), total Akt, p-mTOR (Ser2448), total mTOR, p-FoxO-1 (Ser253), total FoxO-1, GLUT-2, and PDX-1 of islets from RIP_cre+ Pten+/+ and RIP_cre_+ _Pten_fl/fl mice fed either chow or HFD (n = 3). *P < 0.05. Scale bar, 50 μm. Results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 6.
RIP_cre_+ Pten_fl/fl Leprdb/db mice exhibited comparable weight gain and normal glucose tolerance despite being insulin resistant with normal β-cell function. A: Weight of wild-type (WT), RIP_cre+ Pten+/+ Leprdb/db (db/db), and RIP_cre_+ Pten_fl/fl Leprdb/db (−/−; db/db) mice at 2 and 7 months of age (n >7). B: Fed blood glucose of wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice from 2 to 7 months of age (n >7). C: Glucose tolerance test of wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice at 7 months of age (n >7). D: Insulin tolerance test of wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice at 7 months of age (n >7). E: In vivo glucose-stimulated insulin secretion of wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice at 7 months of age (n = 3). F and G: Insulin secretion per 60 islets during perifusion analysis (F) and quantification of area under the curve (G) of wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice (n = 3). *P < 0.05 for RIP_cre+ Pten_fl/fl Leprdb/db mice compared with RIP_cre+ Pten+/+ Leprdb/db mice or as indicated; φ_P_ < 0.05 for both RIP_cre_+ Pten+/+ Leprdb/db and RIP_cre_+ _Pten_fl/fl Leprdb/db mice compared with wild-type mice. Results are presented as mean ± SE.
FIG. 7.
RIP_cre_+ Pten_fl/fl Leprdb/db mice exhibit islet hypertrophy with normal islet architecture and enhanced PI3K signaling. A: Immunofluorescent staining of insulin/glucagon, and immunohistochemical staining of synaptophysin, p-Akt (Ser473), total Akt, GLUT2, and PDX-1 in wild-type (WT), RIP_cre+ Pten+/+ Leprdb/db (db/db), and RIP_cre_+ Pten_fl/fl Leprdb/db (−/−; db/db) mice at 7 months of age. B: Quantification of islet area in wild type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db at 7 months of age (n = 4). C: Percentage of Ki67 positive cells in wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ Pten_fl/fl Leprdb/db mice at 7 months of age (n = 4). D: Western blot (left panel) and quantification (right panel) of p-Akt (Ser473), total Akt, p-mTOR (Ser2448), total mTOR, p-FoxO-1 (Ser253), total FoxO-1, GLUT-2, and PDX-1 of islets from wild-type, RIP_cre+ Pten+/+ Leprdb/db, and RIP_cre_+ _Pten_fl/fl Leprdb/db mice at 7 months of age (n = 3). *P < 0.05. Scale bar, 500 μm (A, top panel); 50 μm (A, rows 2 to 6). Results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 8.
HFD-fed RIP_cre_+ Pten_fl/fl and RIP_cre+ Pten_fl/fl Leprdb/db mice showed intact islet integrity and uncompromised response to gamma irradiation. A: Laminin and β-catenin staining demonstrated intact basement membrane and cell-to-cell adhesion in HFD-fed (9 months of age) RIP_cre+ Pten_fl/fl (−/−) and RIP_cre+ Pten_fl/fl Leprdb/db (−/−; db/db) (9 months of age) mice. B: Transcripts levels of p53-related gene including Bax, Mdm2, and p21 during quantitative PCR of RIP_cre+ Pten+/+ (+/+) and RIP_cre_+ Pten_fl/fl islets with or without 30Gy gamma irradiation (n = 2). C: Western blotting (left panel) and quantification (right panel) of p-p53 (Ser392), total p53, cleaved MDM2, and total MDM2 in RIP_cre+ Pten+/+ and RIP_cre_+ _Pten_fl/fl islets with or without 30Gy gamma irradiation (n = 2). *P < 0.05. Scale bar, 50 μm. Results are presented as mean ± SE. (A high-quality digital representation of this figure is available in the online issue.)
Similar articles
- Overexpression of microRNA-26a protects against deficient β-cell function via targeting phosphatase with tensin homology in mouse models of type 2 diabetes.
Song Y, Jin D, Jiang X, Lv C, Zhu H. Song Y, et al. Biochem Biophys Res Commun. 2018 Jan 1;495(1):1312-1316. doi: 10.1016/j.bbrc.2017.11.170. Epub 2017 Dec 2. Biochem Biophys Res Commun. 2018. PMID: 29191656 - Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans.
Bensellam M, Shi YC, Chan JY, Laybutt DR, Chae H, Abou-Samra M, Pappas EG, Thomas HE, Gilon P, Jonas JC. Bensellam M, et al. Diabetologia. 2019 Dec;62(12):2273-2286. doi: 10.1007/s00125-019-05008-3. Epub 2019 Oct 17. Diabetologia. 2019. PMID: 31624901 - Distinct in vivo roles of caspase-8 in beta-cells in physiological and diabetes models.
Liadis N, Salmena L, Kwan E, Tajmir P, Schroer SA, Radziszewska A, Li X, Sheu L, Eweida M, Xu S, Gaisano HY, Hakem R, Woo M. Liadis N, et al. Diabetes. 2007 Sep;56(9):2302-11. doi: 10.2337/db06-1771. Epub 2007 Jun 11. Diabetes. 2007. PMID: 17563067 - Role of VEGF-A in pancreatic beta cells.
Watada H. Watada H. Endocr J. 2010;57(3):185-91. doi: 10.1507/endocrj.k09e-035. Epub 2010 Feb 24. Endocr J. 2010. PMID: 20179357 Review. - GLIS3: A Critical Transcription Factor in Islet β-Cell Generation.
Scoville DW, Jetten AM. Scoville DW, et al. Cells. 2021 Dec 9;10(12):3471. doi: 10.3390/cells10123471. Cells. 2021. PMID: 34943978 Free PMC article. Review.
Cited by
- A new role for the brain in metabolic control.
Carvalheira JB, Odegaard JI, Chawla A. Carvalheira JB, et al. Nat Med. 2014 May;20(5):472-3. doi: 10.1038/nm.3556. Nat Med. 2014. PMID: 24804753 No abstract available. - Tet2 Controls the Responses of β cells to Inflammation in Autoimmune Diabetes.
Rui J, Deng S, Perdigoto AL, Ponath G, Kursawe R, Lawlor N, Sumida T, Levine-Ritterman M, Stitzel ML, Pitt D, Lu J, Herold KC. Rui J, et al. Nat Commun. 2021 Aug 20;12(1):5074. doi: 10.1038/s41467-021-25367-z. Nat Commun. 2021. PMID: 34417463 Free PMC article. - β-Cell Insulin Resistance Plays a Causal Role in Fat-Induced β-Cell Dysfunction In Vitro and In Vivo.
Ivovic A, Yung JHM, Oprescu AI, Vlavcheski F, Mori Y, Rahman SMN, Ye W, Eversley JA, Wheeler MB, Woo M, Tsiani E, Giacca A. Ivovic A, et al. Endocrinology. 2024 Mar 29;165(5):bqae044. doi: 10.1210/endocr/bqae044. Endocrinology. 2024. PMID: 38578954 Free PMC article. - Good news for the ageing beta cell.
Bender A, Stewart AF. Bender A, et al. Diabetologia. 2014 Feb;57(2):265-9. doi: 10.1007/s00125-013-3114-7. Epub 2013 Nov 21. Diabetologia. 2014. PMID: 24257895 Free PMC article. No abstract available. - Survivin is required for beta-cell mass expansion in the pancreatic duct-ligated mouse model.
Wu X, Zhang Q, Wang X, Zhu J, Xu K, Okada H, Wang R, Woo M. Wu X, et al. PLoS One. 2012;7(8):e41976. doi: 10.1371/journal.pone.0041976. Epub 2012 Aug 1. PLoS One. 2012. PMID: 22870272 Free PMC article.
References
- Marchetti P, Del Guerra S, Marselli L, Lupi R, Masini M, Pollera M, Bugliani M, Boggi U, Vistoli F, Mosca F, Del Prato S: Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab 2004;89:5535–5541 - PubMed
- Mitrakou A, Kelley D, Mokan M, Veneman T, Pangburn T, Reilly J, Gerich J: Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N Engl J Med 1992;326:22–29 - PubMed
- Porte D, Jr, Kahn SE: β-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes 2001;1(Suppl.):S160–S163 - PubMed
- Kahn SE: β cell failure: causes and consequences. Int J Clin Pract 2001;September:13–18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous