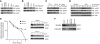HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans - PubMed (original) (raw)
. 2010 Oct;120(10):3578-93.
doi: 10.1172/JCI42442. Epub 2010 Sep 13.
Priya Koppikar, Tony Taldone, Omar Abdel-Wahab, Nathan West, Neha Bhagwat, Eloisi Caldas-Lopes, Kenneth N Ross, Mithat Gönen, Alex Gozman, James H Ahn, Anna Rodina, Ouathek Ouerfelli, Guangbin Yang, Cyrus Hedvat, James E Bradner, Gabriela Chiosis, Ross L Levine
Affiliations
- PMID: 20852385
- PMCID: PMC2947224
- DOI: 10.1172/JCI42442
HSP90 is a therapeutic target in JAK2-dependent myeloproliferative neoplasms in mice and humans
Sachie Marubayashi et al. J Clin Invest. 2010 Oct.
Abstract
JAK2 kinase inhibitors were developed for the treatment of myeloproliferative neoplasms (MPNs), following the discovery of activating JAK2 mutations in the majority of patients with MPN. However, to date JAK2 inhibitor treatment has shown limited efficacy and apparent toxicities in clinical trials. We report here that an HSP90 inhibitor, PU-H71, demonstrated efficacy in cell line and mouse models of the MPN polycythemia vera (PV) and essential thrombocytosis (ET) by disrupting JAK2 protein stability. JAK2 physically associated with both HSP90 and PU-H71 and was degraded by PU-H71 treatment in vitro and in vivo, demonstrating that JAK2 is an HSP90 chaperone client. PU-H71 treatment caused potent, dose-dependent inhibition of cell growth and signaling in JAK2 mutant cell lines and in primary MPN patient samples. PU-H71 treatment of mice resulted in JAK2 degradation, inhibition of JAK-STAT signaling, normalization of peripheral blood counts, and improved survival in MPN models at doses that did not degrade JAK2 in normal tissues or cause substantial toxicity. Importantly, PU-H71 treatment also reduced the mutant allele burden in mice. These data establish what we believe to be a novel therapeutic rationale for HSP90 inhibition in the treatment of JAK2-dependent MPN.
Figures
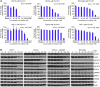
Figure 1. Effects on viability and signaling in MPN mutant cell lines treated with PU-H71.
(A) Cells bearing mutations that result in constitutive activation of the JAK-STAT signaling pathway (JAK2V617F [V617F] and MPLW515L [W515L]) have a lower IC50 compared with that of Ba/F3 cells bearing BCR-Abl. Similarly, UKE-1 cells bearing JAK2V617F are more growth inhibited by PU-H71 than either KU812 (BCR-ABL) or THP-1 (JAK2 wild-type [JAK2WT]). (B) Western blots reveal a dose-dependent downmodulation of JAK2 and signaling intermediates in the JAK-STAT pathway after treatment with PU-H71 for 16 hours in Ba/F3 isogenic cell lines.
Figure 2. JAK2 associates with HSP90 and is degraded via the proteasomal pathway.
(A) Ba/F3 isogenic and human leukemic cell lysates were immunoprecipitated with JAK2 and then probed for pJAK2, HSP90, and JAK2. (B) In a reciprocal experiment, cell lysates were incubated with PU-H71–conjugated beads and then blotted for JAK2 and HSP90. (C) THP-1 and UKE-1 cells were incubated with DMSO or 5 μM TG101348, a JAK2 inhibitor, for 4 hours, and then lysates were immunoprecipitated with JAK2, followed by Western blotting for pJAK2, HSP90, and JAK2. Association with HSP90 is not dependent upon phosphorylation. (D) Titration of PU-H71 beads does not change the kinetics of binding of PU-H71–conjugated beads with JAK2 or HSP90. Raf1, a known client protein of HSP90, is also shown. (E) Cycloheximide, a protein synthesis inhibitor, was used to determine the half-life of JAK2 protein. UKE-1 cells were pretreated with cycloheximide, along with either DMSO or 500 nM PU-H71, and harvested at different time points. Protein degradation began as early as 1 hour with PU-H71 treatment and was completely depleted after 8 hours of treatment. (F) UKE-1 cells were pretreated with 5 μM MG-132, before a 16-hour incubation with DMSO or 500 nM PU-H71. JAK2 expression was observed in the insoluble fraction with MG-132 treatment, showing that with PU-H71 treatment, JAK2 is degraded via the proteasomal pathway.
Figure 3. Combined inhibition of HSP90 and JAK2 impairs MPN cell proliferation.
Pairwise dose-response data and isobologram synergy plots are presented for (A) PU-H71 and TG101348, (B) PU-H71 and Jak Inhibitor I, and (C) TG101348 and Jak Inhibitor I. Data are presented as mean measurements of the normalized proliferation of the UKE-1 cell line, treated for 72 hours, as determined by ATP content. Dose-response data are derived from a linear regression of 8 experimental replicates, plotted also with mean ± SD (GraphPad Prism). Normalized isobolograms are derived from matrix proliferation data analyzed using the median-effect principle of Chou and Talalay (Calcusyn). Error bars reflect SD calculated from 8 experimental replicates. The diagonal lines represent lines of additivity. Dots indicate paired values of drug concentrations assessed for synergism, using the median effect principle of Chou and Talalay. Additive and synergistic effects of the compounds are observed across a broad range of concentrations.
Figure 4. Inhibition of HSP90 by PU-H71 modulates the STAT5A transcriptional program.
(A) Gene expression profiling was performed on the human leukemic cell line, UKE-1. UKE-1 cells were treated for 8 hours with DMSO, 250 nM PU-H71, 2 μM JAK Inhibitor I (JAKi), or a combination of both. The heat map presents the top 20 genes that discriminate between the treatment condition and control vehicle-treated samples. (B–D) Genes with increased expression are presented in red, whereas genes with decreased expression are shown in blue. GSEA was performed with each treatment condition to assess for modulation of (B) STAT5A target genes (C) HSF1 target genes and (D) genes modulated by exposure to the HSP90 inhibitor, 17-AAG. Graphical data represent enrichment scores across each genome-wide transcriptional profile and are annotated with normalized enrichment scores (NESs) and P values.
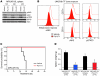
Figure 5. PU-H71 degrades JAK2 in vivo, reduces myeloproliferation, and improves survival.
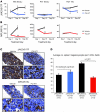
(A and B) Initial pharmacodynamic study of MPLW515L- and JAK2V617F-transduced spleen and bone marrow cells. (A) A single dose of 75 mg/kg PU-H71 for 12, 24, and 48 hours resulted in reduction in JAK2 and pSTAT5 levels, while actin is shown as loading control. (B) PU-H71–treated whole JAK2V617F bone marrow cells as well as the CD71 and CD11 fractions have decreased JAK2 and pSTAT5 expression in comparison with vehicle-treated JAK2V617F bone marrow when measured by flow cytometry. (C) Treatment with PU-H71 resulted in a significant increase in survival of MPLW515L mice compared with vehicle-treated MPLW515L mice as shown by the Kaplan-Meier survival curve (P < 0.0004, log-rank test). (D) Spleen weights of PU-H71–treated MPLW515L or JAK2V617F mice are significantly lower than those of vehicle-treated mice sacrificed at the same time (*P < 0.01). The horizontal line indicates the average weight of the normal BALB/c female mouse spleen at 8 weeks.
Figure 6. Lineage-specific reduction in myeloproliferation with PU-H71 treatment.
(A) White blood cell count and hematocrit (HCT) values of PU-H71–treated JAK2V617F mice are lower in comparison with those of vehicle-treated mice, but there is no difference in the platelet (PLT) counts between both groups. *P < 0.005. (B) PU-H71–treated MPLW515L mice demonstrate reduced white blood cell and platelet counts, with unchanged hematocrit values over time. **P < 0.01. (C) Ter119 expression in PU-H71–treated bone marrow is reduced in comparison with vehicle-treated JAK2V617F bone marrow, while there are no significant differences between either vehicle- or drug-treated MPLW515L marrow. Original magnification, ×200 (top row); ×600 (bottom row). (D) PU-H71 significantly decreased the average number of megakaryocytes in spleens of MPLW515L mice (P < 0.001) but not JAK2V617F-treated mice.
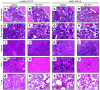
Figure 7. Histopathology of vehicle- and PU-H71–treated bone marrow, spleen, liver, and lung tissue.
(A, B, E, and F) Tissue harvested at the same time demonstrated a slight decrease in cellularity between vehicle and PU-H71–treated JAK2V617F bone marrow. (C,D, G, and H) MPLW515L bone marrow showed a decrease in cellularity between vehicle- and PU-H71–treated tissue. (I and J) PU-H71–treated JAK2V617F spleen showed a reduction in myeloid infiltration as compared with vehicle-treated spleen. (K and L) PU-H71–treated MPLW515L spleen, on the other hand, showed fewer numbers of megakaryocytes (indicated by arrows). (M–P) There was extramedullary hematopoiesis in vehicle-treated JAK2V617F and MPLW515L liver that was reduced with PU-H71 treatment. (Q–T) Further, lung histopathology revealed an increase in neutrophils and extramedullary hematopoiesis in vehicle-treated JAK2V617F and MPLW515L mice that was decreased with PU-H71 treatment. Original magnification, ×200 (first, fourth, and fifth rows); ×400 (third row); ×600 (second row). mJAK2, mouse JAK2; hMPL, human MPL.
Figure 8. PU-H71 retention and allele burden in MPLW515L-transduced BALB/c mice.
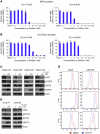
BALB/c mice were transplanted with both untransduced and MPLW515L-transduced bone marrow cells. Mice were sacrificed 2 or 12 hours after a single dose of 75 mg/kg PU-H71. Relevant tissues were harvested for LC-MS/MS analysis and Western blots. (A) LC-MS/MS results for relevant tissues over time. Spleen tissue in both control and MPLW515L mice at both 2 and 12 hours shows rapid uptake by 2 hours but only MPLW515L-specific accumulation of PU-H71 at 12 hours. (B) Western blotting of spleen tissue from control and MPLW515L mice at both 2 and 12 hours shows comparable levels of JAK2 at 2 hours, with a decrease in JAK2 levels in MPLW515L mice at 12 hours. Actin and HSP90 are shown as loading controls. (C) GFP percentage over time in both vehicle and 75 mg/kg PU-H71–treated MPLW515L mice. Initial levels of GFP are similar for both groups; however, over time there is a statistically significant decrease in the PU-H71–treated mice. *P = 0.004.
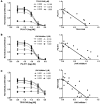
Figure 9. PU-H71 inhibits growth and signaling of JAK2V617F mutant MPN samples.
(A and B) Viability assays of CD34 cells isolated from_JAK2V617F_-positive PV patients and normal cord blood cells show that the _JAK2V617F_-positive mutant cells are more sensitive to growth inhibition by PU-H71 than the CD34-positive cells isolated from normal cord blood. (C and D) Western blot analysis after treatment of the primary samples with either DMSO or 250 nM PU-H71 showed that JAK2 levels in PU-H71–treated patient samples were lower than those in normal cord blood samples. This decrease in JAK2 levels also correlated with increased inhibition of pSTAT5 and increased levels of HSP70 in these samples in comparison with cord blood samples. Total STAT5 and actin are shown as loading controls. (E) Flow cytometry revealed a decrease in both JAK2 and pSTAT5 levels in drug-treated patient samples.
Similar articles
- JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation.
Pandey G, Kuykendall AT, Reuther GW. Pandey G, et al. Blood Cancer J. 2022 Jan 26;12(1):13. doi: 10.1038/s41408-022-00609-5. Blood Cancer J. 2022. PMID: 35082276 Free PMC article. Review. - Improved targeting of JAK2 leads to increased therapeutic efficacy in myeloproliferative neoplasms.
Bhagwat N, Koppikar P, Keller M, Marubayashi S, Shank K, Rampal R, Qi J, Kleppe M, Patel HJ, Shah SK, Taldone T, Bradner JE, Chiosis G, Levine RL. Bhagwat N, et al. Blood. 2014 Mar 27;123(13):2075-83. doi: 10.1182/blood-2014-01-547760. Epub 2014 Jan 27. Blood. 2014. PMID: 24470592 Free PMC article. - Pre-clinical efficacy of PU-H71, a novel HSP90 inhibitor, alone and in combination with bortezomib in Ewing sarcoma.
Ambati SR, Lopes EC, Kosugi K, Mony U, Zehir A, Shah SK, Taldone T, Moreira AL, Meyers PA, Chiosis G, Moore MA. Ambati SR, et al. Mol Oncol. 2014 Mar;8(2):323-36. doi: 10.1016/j.molonc.2013.12.005. Epub 2013 Dec 15. Mol Oncol. 2014. PMID: 24388362 Free PMC article. Clinical Trial. - The Development and Use of Janus Kinase 2 Inhibitors for the Treatment of Myeloproliferative Neoplasms.
Hobbs GS, Rozelle S, Mullally A. Hobbs GS, et al. Hematol Oncol Clin North Am. 2017 Aug;31(4):613-626. doi: 10.1016/j.hoc.2017.04.002. Epub 2017 May 17. Hematol Oncol Clin North Am. 2017. PMID: 28673391 Review. - PU-H71, a novel Hsp90 inhibitor, as a potential cancer-specific sensitizer to carbon-ion beam therapy.
Li HK, Matsumoto Y, Furusawa Y, Kamada T. Li HK, et al. J Radiat Res. 2016 Sep;57(5):572-575. doi: 10.1093/jrr/rrw054. Epub 2016 May 29. J Radiat Res. 2016. PMID: 27242340 Free PMC article.
Cited by
- JAK2 inhibitor persistence in MPN: uncovering a central role of ERK activation.
Pandey G, Kuykendall AT, Reuther GW. Pandey G, et al. Blood Cancer J. 2022 Jan 26;12(1):13. doi: 10.1038/s41408-022-00609-5. Blood Cancer J. 2022. PMID: 35082276 Free PMC article. Review. - Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond.
Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Quintás-Cardama A, et al. Nat Rev Drug Discov. 2011 Feb;10(2):127-40. doi: 10.1038/nrd3264. Nat Rev Drug Discov. 2011. PMID: 21283107 Review. - Combination of PIM and JAK2 inhibitors synergistically suppresses MPN cell proliferation and overcomes drug resistance.
Huang SM, Wang A, Greco R, Li Z, Barberis C, Tabart M, Patel V, Schio L, Hurley R, Chen B, Cheng H, Lengauer C, Pollard J, Watters J, Garcia-Echeverria C, Wiederschain D, Adrian F, Zhang J. Huang SM, et al. Oncotarget. 2014 May 30;5(10):3362-74. doi: 10.18632/oncotarget.1951. Oncotarget. 2014. PMID: 24830942 Free PMC article. - Challenges and Perspectives for Therapeutic Targeting of Myeloproliferative Neoplasms.
Brkic S, Meyer SC. Brkic S, et al. Hemasphere. 2020 Dec 29;5(1):e516. doi: 10.1097/HS9.0000000000000516. eCollection 2021 Jan. Hemasphere. 2020. PMID: 33403355 Free PMC article. Review. - Givinostat: an emerging treatment for polycythemia vera.
Chifotides HT, Bose P, Verstovsek S. Chifotides HT, et al. Expert Opin Investig Drugs. 2020 Jun;29(6):525-536. doi: 10.1080/13543784.2020.1761323. Epub 2020 Jul 21. Expert Opin Investig Drugs. 2020. PMID: 32693648 Free PMC article. Review.
References
- Dameshek W. Some speculations on the myeloproliferative syndromes. Blood. 1951;6(4):372–375. - PubMed
- Baxter EJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous