Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain - PubMed (original) (raw)
Review
Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer's amyloid β-peptide elimination from the brain
Berislav V Zlokovic et al. J Neurochem. 2010 Dec.
Abstract
Low-density lipoprotein receptor-related protein-1 (LRP1), a member of the low-density lipoprotein receptor family, has major roles in the cellular transport of cholesterol, endocytosis of 40 structurally diverse ligands, transcytosis of ligands across the blood-brain barrier, and transmembrane and nuclear signaling. Recent evidence indicates that LRP1 regulates brain and systemic clearance of Alzheimer's disease (AD) amyloid β-peptide (Aβ). According to the two-hit vascular hypothesis for AD, vascular damage precedes cerebrovascular and brain Aβ accumulation (hit 1) which then further amplifies neurovascular dysfunction (hit 2) preceding neurodegeneration. In this study, we discuss the roles of LRP1 during the hit 1 and hit 2 stage of AD pathogenesis and describe a three-level serial LRP1-dependent homeostatic control of Aβ clearance including (i) cell-surface LRP1 at the blood-brain barrier and cerebrovascular cells mediating brain-to-blood Aβ clearance (ii) circulating LRP1 providing a key endogenous peripheral 'sink' activity for plasma Aβ which prevents free Aβ access to the brain, and (iii) LRP1 in the liver mediating systemic Aβ clearance. Pitfalls in experimental Aβ brain clearance measurements with the concurrent use of peptides/proteins such as receptor-associated protein and aprotinin are also discussed. We suggest that LRP1 has a critical role in AD pathogenesis and is an important therapeutic target in AD.
© 2010 The Authors. Journal of Neurochemistry © 2010 International Society for Neurochemistry.
Figures
Fig. 1
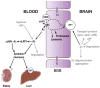
LRP1 schematic structure and ligands. (a) The extracellular heavy α-chain (515 kDa) of LRP1 containing four ligand binding domains (clusters I-IV) is non-covalently coupled to the transmembrane and cytoplasmic light β-chain (85 kDa). β-secretase (BACE) cleaves the N-terminal extracellular domain of LRP1 releasing soluble LRP1 (sLRP1) which circulates in plasma. γ-secretase cleaves the intracellular domain of LRP1 (LRP1-ICD) at the plasma membrane that is translocated from the plasma membrane to the nucleus. EGF, epidermal growth factor; LRP1-CTF, LRP1 C-terminal fragment; Green regions in LRP1-CTF denote two NPXY motifs, the distal NPXY motif overlaps with an YXXL internalization motif. (b) Structurally diverse ligands which bind to clusters II and IV within the extracellular domain of LRP1.
Fig. 2
Schematic diagram illustrating the key role of LRP1 in the three-step serial clearance mechanism mediating Alzheimer's amyloid β-peptide (Aβ) elimination from the brain and the blood. Step 1: The cell surface LRP1 at the abluminal side of the blood-brain barrier (BBB) binds Aβ from the brain interstitial fluid initiating its transcytosis across the BBB to blood. Apolipoprotein J (apoJ), apoE2, apoE3 and apoE4 and α2-macroglobulin (α2M) influence differentially LRP1-mediated Aβ clearance at the BBB. Aβ is generated from Aβ precursor protein (APP) by all types of cells within the neurovascular unit. Degradation indicates enzymatic clearance of Aβ by different enzymes such as for example neprilysin and insulin degrading enzyme. TJ, the tight junctions between brain endothelial cells form an anatomical barrier for transport exchanges of solutes between blood and brain and vice versa, brain and blood. Step 2: β-secretase (BACE) in many organs including the BBB cleaves the N-terminal extracellular domain of LRP1 releasing soluble LRP1 (sLRP1) in the circulation. Circulating sLRP1 provides a key endogenous peripheral ‘sink’ activity for Aβ. sLRP1 normally binds 70-90% of plasma Aβ40 and Aβ42 preventing free Aβ access to the brain. Systemic APP indicates Aβ generation by different peripheral organs and its secretion back into the circulation. Step 3: The cell surface LRP1 in the liver mediates systemic clearance of sLRP1-Aβ complexes and free Aβ ultimately eliminating Aβ from the body. In addition, kidney removes sLRP1-Aβ complexes and Aβ. Whether LRP1 in the kidney can also mediate sLRP1-Aβ and Aβ systemic clearance as in the liver in presently unknown.
Fig. 3
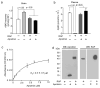
Levels of human Aβ40 in the brain (a) and plasma (b) 30 min after microinjection of human Aβ40 (40 nM) and 14C-inulin (0.023 μCi) into the mouse caudate nucleus in the presence and absence of RAP (5 μM) with and without aprotinin (8.6 μM). Human unlabeled Aβ40 peptide levels in the brain and plasma were determined by using human-specific ELISA, as described (Bell et. al. 2007). The brain sample used for analysis was approximately 15 mg of tissue adjacent to the site of microinjection in the caudate nucleus as described (Bell et al. 2007). In (a) and (b), values are mean ± SEM from 3 to 5 independent experiments. (c) Binding of aprotinin to immobilized human RAP, by ELISA. Briefly, 5 μg/mL repurified human recombinant RAP (Oxford Biomedical Research, Oxford, MI, USA) was coated on microtiter plate and wells were blocked with 1% BSA. Varying concentrations of aprotinin (Sigma, St Louis, MO, USA) were added to the wells and incubated for 1 h at 25°C. Bound aprotinin was detected by mouse anti-aprotinin antibody (Abcam, Cambridge, MA, USA), followed by goat anti-mouse HRP conjugate (Bio-Rad Laboratories, Hercules, CA, USA). The reaction was developed using tetramethyl benzidine substrate (TMB; KPL), stopped with 1M HCl and quantified at 450 nm. Values are mean ± s.e.m. from 3 independent experiments. (d) Formation of RAP and aprotinin complexes detected by the Western blot analysis for aprotonin and RAP. Aprotinin (2 μM; Sigma) was incubated with human recombinant RAP (2 μM; Oxford Biomedical Research) for 1 h at 37°C in PBS and the complex formation was confirmed by cross-linking with Bis(sulfosuccinimidyl)suberate (BS3; Pierce, Rockford, IL, USA) followed by 4-12% SDS-PAGE separation of proteins under non-reducing conditions and Western blot analysis for aprotinin using mouse anti-aprotinin (Abcam) antibody and for RAP using mouse anti-RAP (Oxford Biomedical Research). Representative WB analysis from 3 independent experiments were shown.
Similar articles
- Low-density lipoprotein receptor-related protein 1: a physiological Aβ homeostatic mechanism with multiple therapeutic opportunities.
Sagare AP, Deane R, Zlokovic BV. Sagare AP, et al. Pharmacol Ther. 2012 Oct;136(1):94-105. doi: 10.1016/j.pharmthera.2012.07.008. Epub 2012 Jul 20. Pharmacol Ther. 2012. PMID: 22820095 Free PMC article. Review. - Astrocytic LRP1 Mediates Brain Aβ Clearance and Impacts Amyloid Deposition.
Liu CC, Hu J, Zhao N, Wang J, Wang N, Cirrito JR, Kanekiyo T, Holtzman DM, Bu G. Liu CC, et al. J Neurosci. 2017 Apr 12;37(15):4023-4031. doi: 10.1523/JNEUROSCI.3442-16.2017. Epub 2017 Mar 8. J Neurosci. 2017. PMID: 28275161 Free PMC article. - Inhibition of ADAM10 promotes the clearance of Aβ across the BBB by reducing LRP1 ectodomain shedding.
Shackleton B, Crawford F, Bachmeier C. Shackleton B, et al. Fluids Barriers CNS. 2016 Aug 8;13(1):14. doi: 10.1186/s12987-016-0038-x. Fluids Barriers CNS. 2016. PMID: 27503326 Free PMC article. - Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease.
Deane R, Zlokovic BV. Deane R, et al. Curr Alzheimer Res. 2007 Apr;4(2):191-7. doi: 10.2174/156720507780362245. Curr Alzheimer Res. 2007. PMID: 17430246 Review. - LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer's amyloid-β.
Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. Kanekiyo T, et al. J Neurosci. 2012 Nov 14;32(46):16458-65. doi: 10.1523/JNEUROSCI.3987-12.2012. J Neurosci. 2012. PMID: 23152628 Free PMC article.
Cited by
- Clearance systems in the brain-implications for Alzheimer disease.
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Tarasoff-Conway JM, et al. Nat Rev Neurol. 2015 Aug;11(8):457-70. doi: 10.1038/nrneurol.2015.119. Epub 2015 Jul 21. Nat Rev Neurol. 2015. PMID: 26195256 Free PMC article. Review. - Insoluble Vascular Amyloid Deposits Trigger Disruption of the Neurovascular Unit in Alzheimer's Disease Brains.
Soto-Rojas LO, Campa-Córdoba BB, Harrington CR, Salas-Casas A, Hernandes-Alejandro M, Villanueva-Fierro I, Bravo-Muñoz M, Garcés-Ramírez L, De La Cruz-López F, Ontiveros-Torres MÁ, Gevorkian G, Pacheco-Herrero M, Luna-Muñoz J. Soto-Rojas LO, et al. Int J Mol Sci. 2021 Apr 1;22(7):3654. doi: 10.3390/ijms22073654. Int J Mol Sci. 2021. PMID: 33915754 Free PMC article. - How Do Meningeal Lymphatic Vessels Drain the CNS?
Raper D, Louveau A, Kipnis J. Raper D, et al. Trends Neurosci. 2016 Sep;39(9):581-586. doi: 10.1016/j.tins.2016.07.001. Epub 2016 Jul 25. Trends Neurosci. 2016. PMID: 27460561 Free PMC article. Review. - The Role of Glia in Wilson's Disease: Clinical, Neuroimaging, Neuropathological and Molecular Perspectives.
Gromadzka G, Wilkaniec A, Tarnacka B, Hadrian K, Bendykowska M, Przybyłkowski A, Litwin T. Gromadzka G, et al. Int J Mol Sci. 2024 Jul 9;25(14):7545. doi: 10.3390/ijms25147545. Int J Mol Sci. 2024. PMID: 39062788 Free PMC article. Review. - Low-Density Lipoprotein Receptor-Related Protein 1 as a Potential Therapeutic Target in Alzheimer's Disease.
Petralla S, Panayotova M, Franchina E, Fricker G, Puris E. Petralla S, et al. Pharmaceutics. 2024 Jul 17;16(7):948. doi: 10.3390/pharmaceutics16070948. Pharmaceutics. 2024. PMID: 39065645 Free PMC article. Review.
References
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer's disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. - PubMed
- Bading JR, Yamada S, Mackic JB, Kirkman L, Miller C, Calero M, Ghiso J, Frangione B, Zlokovic BV. Brain clearance of Alzheimer's amyloid-beta40 in the squirrel monkey: a SPECT study in a primate model of cerebral amyloid angiopathy. J Drug Target. 2002;10:359–368. - PubMed
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 NS034467/NS/NINDS NIH HHS/United States
- R37AG023084/AG/NIA NIH HHS/United States
- R37 AG023084/AG/NIA NIH HHS/United States
- R37NS34467/NS/NINDS NIH HHS/United States
- R01 NS034467/NS/NINDS NIH HHS/United States
- R37 NS034467-12/NS/NINDS NIH HHS/United States
- R37 AG023084-08/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous