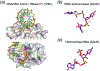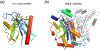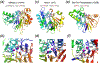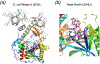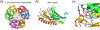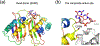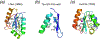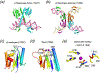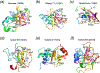Nucleases: diversity of structure, function and mechanism - PubMed (original) (raw)
Review
Nucleases: diversity of structure, function and mechanism
Wei Yang. Q Rev Biophys. 2011 Feb.
Abstract
Nucleases cleave the phosphodiester bonds of nucleic acids and may be endo or exo, DNase or RNase, topoisomerases, recombinases, ribozymes, or RNA splicing enzymes. In this review, I survey nuclease activities with known structures and catalytic machinery and classify them by reaction mechanism and metal-ion dependence and by their biological function ranging from DNA replication, recombination, repair, RNA maturation, processing, interference, to defense, nutrient regeneration or cell death. Several general principles emerge from this analysis. There is little correlation between catalytic mechanism and biological function. A single catalytic mechanism can be adapted in a variety of reactions and biological pathways. Conversely, a single biological process can often be accomplished by multiple tertiary and quaternary folds and by more than one catalytic mechanism. Two-metal-ion-dependent nucleases comprise the largest number of different tertiary folds and mediate the most diverse set of biological functions. Metal-ion-dependent cleavage is exclusively associated with exonucleases producing mononucleotides and endonucleases that cleave double- or single-stranded substrates in helical and base-stacked conformations. All metal-ion-independent RNases generate 2',3'-cyclic phosphate products, and all metal-ion-independent DNases form phospho-protein intermediates. I also find several previously unnoted relationships between different nucleases and shared catalytic configurations.
Figures
Fig. 1
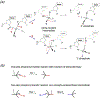
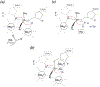
Adjacent nucleotides in RNA and DNA are linked by 3´ and 5´ O-P phosphodiester bonds. a. Nucleases degrade nucleic acid by breaking either one of these two bonds labeled as a and b. A nucleophile attacking in-line from the 5´ side breaks the 3´ O-P bond and produces 5´-phosphate and 3´-OH. Alternatively, a nucleophile attacking the scissile phosphate from the 3´ side breaks the 5´ O-P bond and produces 3´-phosphate and 5´ -OH. b. The SN2 type associative reaction inverts the stereo configuration of phosphate. A two-step trans-esterification reaction can set the stereochemistry back to the original.
Fig. 2
Phosphodiester bond cleavage. a. Nucleases can attack a scissile phosphate in the middle of nucleic acids (endo) or at the 5´ or 3´ ends (exo). The nucleophiles can be the hydroxyl groups of water molecules, Ser or Tyr sidechains, hydroxyls of ribose (2´ or 3’) or deoxyribose (3´) in RNA and DNA, or free nucleophiles. PLD- family (phospholipase D) nucleases use the imidazole of His as the nucleophile. When a protein sidechain serves as a nucleophile, the phosphodiester bond to be cleaved is transferred to the protein. Similarly, when the hydroxyl of a nucleotide or nucleic acid serves as the nucleophile, it is transferred to the nucleic acid. Both are indicated as R. b. DNA transposition by cut-and-paste mechanism. The donor DNA uses its cleaved 3´-OH to attack a phosphate in a target DNA. Thus the donor DNA is inserted into the target in this strand transfer reaction. RNA splicing is similar. The cleavage of the 5´ exon-intron junction frees the 3´-OH of the first exon. This 3´-OH than attacks the 3´ exon-intron junction and results in a linked two exons and free intron. c. DNA Hairpin formation. When a 3´ -OH of a duplex DNA attacks a phosphate of its complementary strand, the two strands of that duplex are linked and become one. d. Type II topoisomerization. Both strands of one DNA segment are cleaved and form covalent bonds with a dimeric (or tetrameric) topoisomerase. The second DNA segment shown in light blue passes through the cleaved segment thus changing the linking number and topology. The cleaved DNA is then religated and the topoisomerase regenerated. e. The 2´ -OH immediate adjacent to a scissile phosphate can serve as a nucleophile and hydrolysis of 5´ O-P bond leads to 2´,3´ cyclic phosphate and 5´-OH. f. RNase PH and PNPase are 3´ exonucleases. They use inorganic phosphate as nucleophiles and degrade RNA to nucleotide diphosphates.
Fig. 3
Double versus single stranded nucleic acid substrate. a. To cleave a double helix the nucleophile can attack from outside of the duplex only. Show here is an RNA/DNA hybrid in a mixed A and B form, which is approached by RNase H1 from the minor groove side. A water molecule is coordinated by the Mg2+ ion in the active site to attack the phosphate from the 5´ side and produce a 5´ phosphate and 3´ -OH. b. A scissile phosphate in pre-tRNA to be cleaved by tRNA endonuclease using the 2´-OH as the nucleophile. The nucleotides surrounding the scissile phosphate are not base paired and splayed. The phosphate itself is distorted. c. Self-cleaving hammerhead ribozyme distorts the scissile phosphate in a similar way because the double helix conformation is incompatible with the reaction coordinates of using a 2´-OH to attack the adjacent phosphate.
Fig. 4
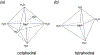
Metal-dependent nucleases most often use Mg2+ and Zn2+ to facilitate phosphodiester bond breakage. a. Diagram of octahedral coordination preferred by Mg2+ and can also be adopted by Zn2+. b. Tetrahedral coordination favored by Zn2+.
Fig. 5
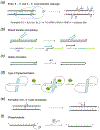
Metal-ion dependent catalysis. a. Two-metal-ion mechanism. The pro-Sp oxygen of a scissile phosphate coordinates both metal ions, one on the 5´ side and the other on the 3´ side. The pro-Rp oxygen is exposed to solvent. When labeling, pro-Sp is abbreviated as Sp and pro-Rp as Rp. b. The three-metal-ion mechanism is a variation of the two-metal-ion mechanism. The scissile phosphate is turned and the pro-Rp oxygen coordinates the two catalytic metal ions and, the pro-Sp oxygen is stabilized by the third divalent cation. c. The one-metal-ion mechanism is an alternative to the two-metal-ion mechanism. One metal ion (A) is eliminated and may be replaced by a positively charged protein sidechain. In the cases where water is the nucleophile, a conserved His often acts as the general base to deprotonate it for nucleophilic attack. Alternatively, Tyr is the nucleophile as in topoisomerases, relaxases and RCR-related recombinases. In all three metal-ion-dependent mechanisms, the nucleophile is always on the 5´ side, and cleavage results in 5´-phosphate and 3´-OH.
Fig. 6
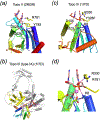
The DEDD superfamily consists of DnaQ-like exonucleases. a. The core of the 3´ - 5´ exonuclease domain of E. coli DNA polymerase I (PDB code is shown in parenthesis) represents the DEDDy nucleases. b. Dimeric TREX1 (2O4G) represents the DEDDh exonucleases. Proteins are shown in ribbon diagrams. One subunit of each enzyme is shown in rainbow colors from the blue N- to red C-terminus. Divalent cations are shown as purple spheres, and the nucleophile water as a red sphere. The sidechains of the conserved DEDDy/h motifs and nucleic acid/nucleotide are shown in sticks with oxygen in red and nitrogen in blue. All DEDD-family members use the two-metal-ion mechanism as represented in the enzyme-nucleic acid or nucleotide complex structures.
Fig. 7
The RNase H superfamily consists of various endonucleases. a. E. coli RNase H1 is the prototype of this superfamily. b. The active site of B. halodurans RNase H complexed with an RNA/DNA hybrid substrate. c. Tn5 transposase (complexed with a cleaved DNA product) represents the HIV integrase-like recombinases with the DDE motif. d. RNase H2 has the same topology as RNase H1 but the second and fourth conserved carboxylates are located differently. e. The active site of Tth Argonaute, essential for RNAi pathways is similar to that of RNase H1. f. RuvC is a Holliday-junction resolvase. g. The C-terminal nuclease domain of UvrC may use two conserved carboxylates and a His for metal ion coordination and catalysis. h. Monomeric Hsp70 ATPases contain two RNase H-like domains. The duplicated domains are both shown in blue to red color gradients. The Asp residues in the first β strand (D10 and D199) are conserved throughout the Hsp70 family and essential for the ATPase activity. Proteins, nucleic acids and divalent cations are shown in the same scheme as in Fig. 6. The green spheres represent the K+ ions.
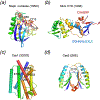
Fig. 8
The DEK superfamily consists of sequence- or structure-specific endonucleases and processive 3´ - 5´ exonucleases. a. The active site of BamHI contains (E)DEE, a variation of DEK. Except for panel b, the region containing the catalytic residues is shown in rainbow colored ribbon diagrams. The rest of the protein is colored grey. Divalent cations and the nucleophilic water are shown in purple and red spheres, respectively. b. Orthogonal views of BamHI-DNA interactions. The two BamHI subunits are shown in grey and light purple. The uneven β hairpins containing the DEK motif are highlighted in the rainbow colors. Only the β hairpins are shown when looking into the DNA major groove. BamHI cleaves DNA to form 4nt 5´ overhangs. The nucleotides containing the scissile phosphate are highlighted in pink sticks. c. Orthogonal views of HincII-DNA interactions. HincII cleaves DNA to blunt ends. The dimerization interface of HincII is nearly perpendicular to that of BamHI when looking down the DNA helical axis. d. E. coli MutH is a sequence- and methyl-specific endonucleaese with the DEK motif. The conserved Lys (K79) bridges the DNA sequence-specific binding in the major groove (shown in blue) and catalytic center approaching the minor groove (shown in pink). The divalent cations (Ca2+) are shown as green spheres. e. The trimeric phage λ exonuclease has a toroidal shape and degrades ssDNA processively. The three subunits are shown in grey, pink and blue ribbon diagrams. The active site region of the grey subunit is highlighted in rainbow colors. f. A zoom-in view of the active site with the eDEK motif. g. RecE, which is a processive exonuclease and promotes DNA recombination, is shown in the same color scheme as BamHI. h. The active site of RecB has the DEK motif with a His at its N-terminus like RecE. i. The Holliday-junction resolvase T7 endonuclease I has the (E)DEK motif like MutH and binds two metal ions. j. The structure specific endonuclease Mus81 and XPF also contain the DEK motif with two conserved carboxylates N-terminal to it. k. Vsr is a sequence-specific endonuclease. The DEK motif may be replaced by DHH, and the two metal ions in the active site are coordinated by a carbonyl oxygen (of T63) in addition to the conserved Asp and scissile phosphate. l. Dom3Z and Rai1 have the conserved DEK motif and can hydrolyze GTP.
Fig. 9
The FEN1 and 5´ - 3´ exonuclease superfamily. a. The active site of the 5´ - 3´ exonuclease domain of Taq DNA polymerase. Four of seven conserved carboxylates (encircled) likely participate in metal-ion binding and catalysis. b. Phage T4 FEN1, previously known as T4 RNase H. The structure was determined in the complex with DNA substrate, but the scissile phosphate is too far away from the catalytic residues in the absence of divalent cations. c. The archaeal FEN1 has the same active site composition as the bacterial and phage orthologues. The bound DNA is far away from the nuclease active site. d. Rat1, which is an RNase, has the same overall structure and conserved active site as archaeal FEN1. e. The PIN domain represents the minimal catalytic core of the nucleases in this superfamily. In all panels, the protein topology is shown in the color gradient from the blue N- to red C-terminus.
Fig. 10
Two nuclease families related to FEN1. a. RecJ is a representative member of the DHH family. The catalytic and DNA binding domain of RecJ are shown in ribbon diagrams. The catalytic domain containing the DHH motif is shown in rainbow colors with the gradient of blue N- to red C-terminus. The rest of RecJ is shown in grey. b. A divalent cation is found in the carboxylate-rich active site in the absence of substrate. c. TOPRIM M5 nuclease family has a βα fold of a central parallel 4-stranded β sheet surrounded by α helices. The catalytic carboxylates are located on the loops linking secondary structures. d. The active site of M5 nuclease. The four carboxylates are shown as sticks.
Fig. 11
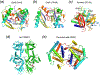
DNase I family. a. The overall structure of DNase I. Directions of strands are indicated in the parenthesis at the foot of the panel and, the strands contributing catalytic residues are highlighted in red. The active site is encircled. b. DNase I with bound substrate. The view is roughly orthogonal to panel a. The two β sheets are related by a pseudo dyad axis lying in the plane of the page and roughly parallel to the strands. c. The active site of DNase I. The pro-Sp oxygens of the scissile phosphate and the neighboring phosphate are encircled in green. Note the distortion of the scissile phosphate. d. Similar distortion of the scissile phosphate is also observed in the APE1-substrate complex. Most of catalytic residues are conserved between DNase I and APE1 except for the alternative His and Tyr as indicated by the black arrowheads. e. The catalytic domain of RNase E upon dimerization is proposed to be similar to DNase I. Both subunits are shown as rainbow-colored ribbon diagrams from blue N- to red C-terminus. The dyad axis between the two subunits is perpendicular to the plane of the page. f. The catalytic domain of a single RNase E subunit. The divalent cation is shown as a purple sphere.
Fig. 12
Nucleases of the metallo-β−lactamase and protein phosphatase (PP2A) families. a. The overall structure of tRNase Z of the metallo-β−lactamase family. Directions of strands in the two β sheets are indicated by the array of arrows. Strands contributing the catalytic residues are highlighted in red. b. The active site of tRNase Z consists of six His and two Asp residues. Two metal ions are bound independent of substrate c. The overall structure of Mre11. It bears significant similarity to tRNase Z in the topology, tertiary structure, active site composition and metal-ion binding. However, the composition and locations of the catalytic residues are different. d. The active site of Mre11 consists of five His, one Asn and two Asp. e. A Ser/Thr phosphatase structure is shown for comparison. Its topology has some differences from tRNase Z and Mre11. f. The active site of the protein phosphatase is nearly superimposable with that of Mre11.
Fig. 13
The LAGLIDADG homing endonucleases. a. The structure of homodimeric I-CeuI in complex with DNA substrate. Each subunit is shown in a rainbow color gradient from blue N- to red C-terminus. The DNA is shown in hot pink ribbon diagrams. The divalent cation bound in the active site is shown as a purple sphere. b. Structure of the monomeric I-SceI with two homologous domains in one polypeptide chain. Each domain is colored in blue to red gradient from N- to C-terminus. The green and purple spheres represent the divalent and monovalent cations, respectively. c. The active site of I-SceI consists of three cations and two overlapping catalytic centers, which are encircled in grey and yellow. The two scissile phosphates and surrounding nucleotides are colored pink (not cleaved) and white (cleaved). The most conserved Asp residues of the LAGLIDA
D
G motif are shown in sticks.
Fig. 14
Bacterial and eukaryotic RNases II. a. E. coli RNase II. The catalytic domain is highlighted in rainbow colors, and the remaining domains are shown in grey. The active site is marked by four conserved Asp residues and a bound metal ion (purple sphere). b. The active site of Rrp44 in complex with ssRNA. Rrp44 is the yeast homolog of RNase II and is a part of exosome. The four Asp residues are all located on the loop of a β hairpin.
Fig. 15
RNase III and dicers. a. The orthogonal views of a homodimeric RNase III. Each subunit consists of a catalytic domain (rainbow color) and the RNA-binding domain (light blue). The two active sites are marked by the bound divalent cations (purple spheres). The red arrowheads point at the scissile bonds. The catalytic residues and nucleotides flanking the scissile phosphates are shown in sticks. b. A eukaryotic dicer is shown in a similar orientation as in panel a. It contains two RNase III-like domains. The region of the duplicated catalytic domain is shown in rainbow color from the blue N to red C terminus, and the two active sites are encircled in yellow. c. The active site of the bacterial RNase III and the cleaved RNA.
Fig. 16
Archaeal and eukaryotic RNase PH (PNPase). a. The RNase PH core of an archaeal exosome consists of a trimer of Rrp41-Rrp42 heterodimer. The red arrowheads indicate the active sites. b. The catalytically active Rrp41 is shown as rainbow colored ribbon diagrams. The pink sphere represents the Cl- observed in the crystal structure. The two 3´ nucleotides of ssRNA and the residues in the active site are shown as sticks. c. A zoom-in view of the catalytic center as boxed in b. The Cl- mimicking the nucleophile Pi is poised for nucleophilic attack to produce a fresh 3´-OH and NDP.
Fig. 17
Holliday junction resolvase RusA. a. An overall view of RusA homodimer. Each subunit is shown in rainbow colors. The active site residues and bound DNA near one active site are depicted in sticks. b. A close-up view of the active site. Each active site consists of residues from both subunits (one in rainbow color and the other in silver). Hence it is composite. The catalytic sidechains and two nucleotides surrounding the scissile phosphate are shown as sticks.
Fig. 18
Self-splicing introns. a. A schematic drawing of phosphoryl transfer reactions catalyzed by group I introns. G-O: represents a free guanosine with its 2´-O serving as the nucleophile. b. Structure of the catalytic center of Group I introns. The exons to be ligated are shown in yellow and the nucleotides of the intron involved in catalyses are highlighted in pink and hot pink. Two Mg2+ (purple spheres) are jointly coordinated by the scissile phosphate and a backbone phosphate that mimics the sidechain of Asp in protein enzymes. c. A schematic drawing of phosphoryl transfer reactions catalyzed by Group II introns. A-O: represents an internal adenosine with its 2´-O serving as the nucleophile. d. Structure of the catalytic center of a group II intron in complex with ligated exons as the product of splicing. Two metal ions (purple spheres) again are found in the active site.
Fig. 19
Nucleases using the three-metal-ion mechanism. a. The structure of AP endonuclease IV (Endo IV) has a TIM barrel fold. The ribbon diagram of Endo IV is colored according to the secondary structures: red α helices, yellow β strands and green loops. A bound phosphate ion is shown as sticks and three Zn2+ ions are shown as purple spheres. b. The structure of the Endo IV-DNA complex. The protein is shown in rainbow colors from blue N- to red C-terminus. DNA substrate is shown as sticks. The cleavage strand is shown in pink and the other strand in yellow. c. A close-up view of the active site. The scissile phosphate is turned so that both pro-Rp and pro-Sp oxygen atoms are coordinated by metal ions. d. Nuclease P1 consists of mostly α helices and a β hairpin. The structure represents an enzyme-ssDNA product complex. e. A close-up view of the Nuclease P1 active site as boxed in d. In spite of the unrelated structures, the three Zn2+ ions and the coordination ligands are reminiscent of Endo IV.
Fig. 20
Nucleases that may use the two-metal-ion mechanism. a. Staphylococcal nuclease (SNase). Thymidine diphosphate (pdTp, THP) co-crystallized and the catalytic residues are shown in sticks. A Ca2+ ion captured in the structure is shown as a purple sphere. b. The MutL endonuclease activity resides in its C-terminal domain (CTD), The crystal structure of E. coli MutL CTD (known as LC20) is shown in a rainbow-colored ribbon diagram. Conserved sequence motifs surrounding the metal-ion-binding site are shown in matching colors of the ribbon diagram and modeled onto the structure with sidechains shown as sticks. c. Cas1 is a CRISPR nuclease. The dimerization domain is not shown here. One catalytic domain is shown in rainbow colored ribbon diagrams with helices shown as rods. The active site is composed of three carboxylates and one His. A bound Mn2+ is shown as a purple sphere. d. The structure of Cas2. Cas2 is a CRISPR RNase with the ferredoxin fold. Both subunits of the homodimer Cas2 are shown in rainbow color from blue N- to red C-terminus. The active site is located at the dimer interface with the conserved and catalytically essential Asp shown in sticks and labeled.
Fig. 21
The ββα-Me nucleases. a. The overall structure of homing endonuclease I-PpoI. It is homodimeric, and the identical protein subunits are shown in the same color schemes. The catalytic domain is shown in green, DNA recognition domain in blue and the connection region in yellow. The cations in the active sites are shown as purple spheres, and structurally important Zn2+ ions are shown as orange spheres. DNA is shown in hot pink. b. A close-up of the active site. The ββα structural motif is shown in rainbow colors. The scissile phosphate and surrounding nucleotides are shown as yellow sticks. A Na+ mimicking the catalytic divalent cation in this structure is shown in purple. The catalytic residues are shown in sticks and labeled. The potential nucleophilic water is shown as a red sphere. c. T4 endonuclease VII (Endo VII) resolves Holliday junctions. One of the two subunits is shown in rainbow color (from blue N- to red C-terminus) and the other in silver. The lime-green DNA strands are to be cleaved. The Mg2+ ions in the active site are shown as purple spheres, and structural Zn2+ ions as orange spheres. One ββα motif in this panel is colored cyan. d. A close-up view of the active site of Endo VII. The presentaion scheme is same as in panel b. e. The structure of restriction endonuclease Hpy99I in complex with DNA substrate. The two subunits of Hpy99I are shown as pale blue and yellow ribbon diagrams. The DNA-binding modules of both subunits are highlighted in blue, and the twp ββα-Me motifs in green and cyan. The catalytically essential Mg2+ ions are substituted by Na+ ions and shown as purple spheres. Zn2+ ions (orange spheres) play a structural role. DNA is colored pink and the scissile phosphates are indicated in light pink. f. The active site of Hpy99I. g and h. The overall structures of NucA/EndoG and Vvn. The ββα-Me motif in each structure is highlighted in rainbow colors and the metal ion shown as a purple sphere. The remaining protein structures are shown in silver grey. i. The active site (ββα-Me motif) of colicin E7 in complex with DNA substrate. The catalytic Zn2+ is coordinated by three His sidechains and two oxygens of the scissile phosphate. The general base H545 is mutated to Q to stop the cleavage reaction. j. The catalytic domain of CAD. The ββα-Me motif is highlighted as in panels f and g.
Fig. 22
GIY-YIG nucleases. a. I-TevI homing endonuclease. The catalytic domain is shown in rainbow-colored ribbon diagram. b. A close-up view of the GIY-YIG motif, which forms a β hairpin. The conserved Tyr are labeled. With the following α helix, the structure resembles the ββα-Me motif, but the GIY-YIG motif doesn’t bind metal ion. c. The N-terminal domain of UvrC has the GIY-YIG motif. A divalent cation is coordinated by E76 and probably marks the active site. The catalytic residues Y (of YIG), E and N near the C-terminus are conserved in both and are encircled in yellow.
Fig. 23
The HuH nucleases. a. The nuclease domain of Adeno-associated virus (Aav5) Rep shown in rainbow colored ribbon diagrams. The associated metal ion is shown in purple. b. The structure of TraI relaxase (Y16F mutant) representing the mob family. Only two nucleotides surrounding the scissile phosphate of the ssDNA substrate are shown as sticks. c. A close-up view of the TraI active site. Three His sidechains coordinate the Mg2+, which in turn aligns the scissile phosphate for nucleophilic attach by Y16. d. Ribbon diagrams of the DNA transposase (TnpA) of IS608 complexed with a 3´-end cleavage product. TnpA is homodimeric. The two domain-swapped protein subunits are shown in yellow and cyan, and the two DNAs in hot pink. e. A close-up view of the composite TnpA active site. The last nucleotide at the 3´ end is also shown. The metal-chelating His residues (of the HUH motif) are from one subunit. The third metal ligand (Q131) and the nucleophile Y127 are from the other subunit.
Fig. 24
Topoisomerases containing the TOPRIM motif. a. The active site of S. cerevisiae Topo II in complex with dsDNA substrate. The TOPRIM domain is shown in rainbow-colored ribbon diagram. The nucleophile Y782 and its neighbor R781 are supplied in trans by the other subunit of the dimeric Topo II and shown as yellow sticks. The scissile phosphate and surrounding nucleotides are shown as pink sticks. Nitrogen and oxygen atoms are colored blue and red, respectively. A Mg2+ ion (purple sphere) is coordinated by the conserved carboxylates in the TOPRIM domain. b. Ribbon diagrams of E. coli Topo III (Type IA) in complex with ssDNA substrate. The α-helices are represented by cylinders. The TOPRIM domain (aka domain I) is shown in rainbow colors, and the catalytic domain (domain III) carrying the Tyr nucleophile is shown in yellow. The ssDNA substrate in shown as sticks. c. A close-up view of the active site of Topo III. The scissile phosphate, the conserved carboxylates, and the nuclephile Y328 are nearly superimposable with that of Topo II shown in panel a. d. A model of the reaction coordinates of DNA cleavage by type IA and type II topoisomerases. The active sites of the two types of TOPRIM topoisomerase are superimposed. The metal ion observed in Topo II is incorporated in the composite active site and shown as a lilac sphere. A Lys (K8) of Topo III approximately occupies the metal ion A position. The Arg next to the nucleophile (R781 of Topo II or R330 of Topo III) in the catalytic domain further stabilizes the scissile phosphate and neutralizes the developing negative charges.
Fig. 25
Metal-independent Ser recombinases. a. The structure of γδ resolvase dimer complexed with a palindromic DNA cleavage site (site I). Each protein subunit and DNA are shown in different colors. The catalytic residues are shown as sticks. b. The γδ resolvase tetramer complexed with two cleaved DNA site I. Each protein dimer and DNA site are rearranged extensively. c. The catalytic domain of resolvase is shown in rainbow colors from blue N- to red C-terminus. The catalytic residues S10and R68 are shown in sticks. d. The catalytic domain of RecJ. The metal coordinating carboxylates and the DHH motif are shown as sticks. e. Superposition of the catalytic residues of resolvase (blue) and RecJ (yellow).
Fig. 26
Metal-independent RNases of T1 family and colicins. a. Barnase in complex with a substrate mimic d(GGAC). The RNase is shown in rainbow-colored ribbon diagrams. The scissile phosphate and surrounding nucleotides are shown as magenta sticks. The active site residues are shown in sticks and labeled. Hydrogen bonds with the scissile phosphate and the surrounding bases are shown as dashed lines. b. RNase T1 complexed with a phosphorothioate cleavage product. The sulfur atom in the 2´, 3´ cyclophosphrothioate is shown in light yellow. c. Sarcin homolog restrictocin in complex with the mimic of sarcin/ricin loop. Only the active site is shown. The RNA is not positioned for cleavage. d. The ribonuclease domain of colicin E3. The potential active site residues are shown as sticks in the same colors as their Cα atoms. Those, whose mutations don’t decrease the nuclease activity, are shown in silver. e. The nuclease domain of colicin D with the active site residues shown in sticks. f. Colicin E5 complexed with d(GU). Sequence specificity is determined by the numerous hydrogen bonds between the RNase and the bases surrounding the scissile phosphate. The nucleophilic attack would take place as indicated by the black dashed arrowhead if 2´ -OH is present.
Fig. 27
RNase A and related RNases. a. RNase A in complex with d(ATAAG) substrate mimic. RNase A is shown in rainbow-colored ribbon diagrams with the catalytic triad (HKH) shown as sticks. The scissile phosphate is shown in yellow, the surrounding nucleotides in magenta and other nucleotides in pink sticks. b. An example of RNase T2 family, RNase NT, complexed with two GMP. The structure is shown in the same scheme as in panel a. c. A close-up view of the substrate recognition by RNase NT. The two bases surrounding the putative scissile phosphate are sandwiched by aromatic sidechains (shown in yellow). The HKH catalytic triad (shown in green sticks) appears to be conserved, and the two His sidechains serve as general acid and base. d. The overall structure of a homodimeric tRNA splicing endonuclease. Each catalytic unit consists of a catalytic domain (shown in blue and cyan) and an associated unique domain (shown in yellow and steel blue). Two structural units are shown in silver grey and are homologous to the catalytic units (green+yellow or cyan+light) by gene duplication. The four units can be independent polypeptide chains in other orthologs. The pre-tRNA mimic is shown in orange ribbon diagrams. The uncleaved and cleaved scissile phosphates are boxed and labeled as e and f, respectively. e. A close-up view of the active site (green subunit) with uncleaved RNA (substrate). Hydrogen bonds with the scissile phosphate are shown as hot pink dashed lines. RNA substrate is stabilized by cation-π-cation stacking with a neighboring subunit (cyan). f. The active site (cyan subunit) with the cleavage product, 2´,3´-cyclic phosphate.
Fig. 28
Other metal-independent RNases. a. Pyrococcus furiosus Cas6. The two ferredoxin-like domains arranged in a direct repeat are both shown in rainbow-colored ribbon diagrams, from blue N- to red C-terminus. Putative catalytic residues are shown as sticks. b. Thermus thermophilus CasE is related to Cas6 and shown in the same color scheme. Only the Lys near the C-terminal is conserved between Cas6 and CasE. c. Ribbon diagram of XendoU. The protein is in rainbow color from blue N- to red C-terminus. A pseudodyad symmetry relates the two 3-stranded β sheets. A bound phosphate is shown as orange and red sticks. The conserved and catalytic essential residues are also shown and labeled. d. The overall structure of IreI homodimer. The two subunits are shown in green and cyan, and the bound ADPs in the kinase domains are shown in red sticks. The nuclease domains (top) are highlighted with the catalytic residues shown in sticks. e. A close-up view of the putative active site of IreI. Each nuclease domain is shown in rainbow colors. The residues (in one subunit) with strong effects on catalysis are labeled in black, and those of with medium effects in grey.
Fig. 29
Metal-independent ribozymes. a. Hammerhead ribozyme. The RNA is shown in silver ribbon diagrams. The scissile phosphate is shown in yellow, the surrounding nucleotides (C-1 and A+1) in magenta, and the catalytic nucleotides in cyan. A Mg2+ (purple sphere) happens to be coordinated by the scissile phosphate. b. A close-up view of the catalytic center in the hammerhead. The Mg2+ is omitted for clarity. The reaction is stopped because of the G12A mutation, which reduces the effectiveness of the general base. The 2´-OH of G8 is proposed to be the general acid. c. The overall structure of a catalytically active Hairpin ribozyme. The RNA substrate is shown in pink ribbon diagrams, and the others are shown in the same scheme as in panel a. d. Close-up views of the active site of the hairpin ribozyme complexed with a substrate analog (A-1 is 2´-O-methylated), transition state analog (vanadate) and product (2´, 3´ cyclic phosphate).
Fig. 30
Metal-independent DNases of the PLD family. a. The overall structure of Tyrosine dephosphatase (TDP1). TDP1 serves as a paradigm of phosphoryl transferases using His as the nucleophile. Two gene-duplicated domains are shown in green and cyan. The phosphorylated Tyr is shown in magenta, the scissile phosphate in yellow sticks, and remaining oligonucleotides in pink. The catalytic residues are shown in sticks and colored in green and cyan as the Cα atoms. b. A close-up view of the catalytic center. A mimic of the covalent intermediate (vanadate) is shown in yellow. The duplicated His and Lys are directly involved in catalysis. c. The overall structure of S. typ Nuc shown in the orientation similar to TDP1 in panel a. The two identical subunits are both shown in rainbow colors. d. The catalytic domains of dimeric restriction endonuclease BfiI. The catalytic domains of two subunits are shown in green and cyan and oriented similarly as TDP1 and Nuc. Sequence-specific DNA-binding domains are shown in silver grey.
Fig. 31
Metal-independent topoisomerases and Tyr recombinases. a. The overall structure of Human Topo IB. The protein is shown in rainbow-colored ribbon diagrams. The DNA duplex is shown in pink/blue ribbons. The active site is highlighted with the catalytic residues shown in sticks. The nucleophile Y723 (mutated to Phe) is labeled. b. A close-up view of the active site of Topo IB. The reaction is stopped because of the Y723F (shown in pink) mutation. The scissile phosphate is neutralized by Arg and Lys sidechains. c. The overall structure of the Cre recombinase complexed with substrate LoxP site. The tetrameric Cre is shown in four colors and the two LoxP in pink and yellow. The DNA binding domains are above the DNA and the catalytic domains are below. d. A close-up view of the active site of Cre-LoxP. The scissile phosphate is already pre-nicked. e. Superposition of the catalytic residues of Topo IB and Cre.
Fig. 32
PabI family of restriction endonuclease. a. The overall structure of homodimeric PabI. Two subunits are both shown in rainbow colors. The shape is likened to a halfpipe. The catalytically essential Y134 from both subunits are close together at the bottom of the central groove. b. An orthogonal view of PabI. The catalytically essential residues are shown in sticks and labeled.
Similar articles
- Catalytic metal ions and enzymatic processing of DNA and RNA.
Palermo G, Cavalli A, Klein ML, Alfonso-Prieto M, Dal Peraro M, De Vivo M. Palermo G, et al. Acc Chem Res. 2015 Feb 17;48(2):220-8. doi: 10.1021/ar500314j. Epub 2015 Jan 15. Acc Chem Res. 2015. PMID: 25590654 Review. - Second-Shell Basic Residues Expand the Two-Metal-Ion Architecture of DNA and RNA Processing Enzymes.
Genna V, Colombo M, De Vivo M, Marcia M. Genna V, et al. Structure. 2018 Jan 2;26(1):40-50.e2. doi: 10.1016/j.str.2017.11.008. Epub 2017 Dec 7. Structure. 2018. PMID: 29225080 Free PMC article. - Mechanism and cleavage specificity of the H-N-H endonuclease colicin E9.
Pommer AJ, Cal S, Keeble AH, Walker D, Evans SJ, Kühlmann UC, Cooper A, Connolly BA, Hemmings AM, Moore GR, James R, Kleanthous C. Pommer AJ, et al. J Mol Biol. 2001 Dec 7;314(4):735-49. doi: 10.1006/jmbi.2001.5189. J Mol Biol. 2001. PMID: 11733993 - Recruiting Mechanism and Functional Role of a Third Metal Ion in the Enzymatic Activity of 5' Structure-Specific Nucleases.
Donati E, Genna V, De Vivo M. Donati E, et al. J Am Chem Soc. 2020 Feb 12;142(6):2823-2834. doi: 10.1021/jacs.9b10656. Epub 2020 Jan 27. J Am Chem Soc. 2020. PMID: 31939291 Free PMC article. - Single-strand-specific nucleases.
Desai NA, Shankar V. Desai NA, et al. FEMS Microbiol Rev. 2003 Jan;26(5):457-91. doi: 10.1111/j.1574-6976.2003.tb00626.x. FEMS Microbiol Rev. 2003. PMID: 12586391 Review.
Cited by
- Structure of p22 headful packaging nuclease.
Roy A, Cingolani G. Roy A, et al. J Biol Chem. 2012 Aug 10;287(33):28196-205. doi: 10.1074/jbc.M112.349894. Epub 2012 Jun 19. J Biol Chem. 2012. PMID: 22715098 Free PMC article. - Dynamics of the Artemis and DNA-PKcs Complex in the Repair of Double-Strand Breaks.
Watanabe G, Lieber MR. Watanabe G, et al. J Mol Biol. 2022 Dec 15;434(23):167858. doi: 10.1016/j.jmb.2022.167858. Epub 2022 Oct 19. J Mol Biol. 2022. PMID: 36270581 Free PMC article. Review. - Type III-A CRISPR-associated protein Csm6 degrades cyclic hexa-adenylate activator using both CARF and HEPN domains.
Smalakyte D, Kazlauskiene M, F Havelund J, Rukšėnaitė A, Rimaite A, Tamulaitiene G, Færgeman NJ, Tamulaitis G, Siksnys V. Smalakyte D, et al. Nucleic Acids Res. 2020 Sep 18;48(16):9204-9217. doi: 10.1093/nar/gkaa634. Nucleic Acids Res. 2020. PMID: 32766806 Free PMC article. - DNB-based on-chip motif finding: A high-throughput method to profile different types of protein-DNA interactions.
Li Z, Wang X, Xu D, Zhang D, Wang D, Dai X, Wang Q, Li Z, Gu Y, Ouyang W, Zhao S, Huang B, Gong J, Zhao J, Chen A, Shen Y, Dong Y, Zhang W, Xu X, Xu C, Jiang Y. Li Z, et al. Sci Adv. 2020 Jul 31;6(31):eabb3350. doi: 10.1126/sciadv.abb3350. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32789179 Free PMC article. - Two-metal ion mechanism of DNA cleavage by activated, filamentous SgrAI.
Shan Z, Rivero-Gamez A, Lyumkis D, Horton NC. Shan Z, et al. J Biol Chem. 2024 Aug;300(8):107576. doi: 10.1016/j.jbc.2024.107576. Epub 2024 Jul 14. J Biol Chem. 2024. PMID: 39009341 Free PMC article.
References
- ABELSON J, TROTTA CR & LI H (1998). tRNA splicing. J Biol Chem, 273(21), 12685–12688. - PubMed
- ADAMS PL, STAHLEY MR, KOSEK AB, WANG J & STROBEL SA (2004). Crystal structure of a self-splicing group I intron with both exons. Nature, 430(6995), 45–50. - PubMed
- AIZAWA Y, XIANG Q, LAMBOWITZ AM & PYLE AM (2003). The pathway for DNA recognition and RNA integration by a group II intron retrotransposon. Mol Cell, 11(3), 795–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 DK036119-12/ImNIH/Intramural NIH HHS/United States
- Z01 DK036144-01/ImNIH/Intramural NIH HHS/United States
- Z01 DK036145-02/ImNIH/Intramural NIH HHS/United States
- ZIA DK036144-09/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources