Complete structural model of Escherichia coli RNA polymerase from a hybrid approach - PubMed (original) (raw)
Complete structural model of Escherichia coli RNA polymerase from a hybrid approach
Natacha Opalka et al. PLoS Biol. 2010.
Abstract
The Escherichia coli transcription system is the best characterized from a biochemical and genetic point of view and has served as a model system. Nevertheless, a molecular understanding of the details of E. coli transcription and its regulation, and therefore its full exploitation as a model system, has been hampered by the absence of high-resolution structural information on E. coli RNA polymerase (RNAP). We use a combination of approaches, including high-resolution X-ray crystallography, ab initio structural prediction, homology modeling, and single-particle cryo-electron microscopy, to generate complete atomic models of E. coli core RNAP and an E. coli RNAP ternary elongation complex. The detailed and comprehensive structural descriptions can be used to help interpret previous biochemical and genetic data in a new light and provide a structural framework for designing experiments to understand the function of the E. coli lineage-specific insertions and their role in the E. coli transcription program.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
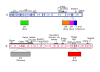
Figure 1. Sequence architecture of the bacterial RNAP large subunits.
The vertical bars represent the primary sequence of the bacterial RNAP β (top, light cyan) and β' (bottom, light pink) subunits.The white boxes indicate sequence regions common to all bacterial RNAPs, as defined by Lane et al. . Important structural features are labeled above the bars . Lineage-specific insertions (labeled according to the nomenclature of Lane et al. are shown below the bars. The color-coding for the large subunits and the lineage-specific insertions shown here is used throughout this article.
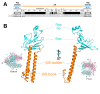
Figure 2. Sequence and structure of Eco RNAP β2-βi4.
(A) Sequence alignment comparing Eco RNAP β2-βi4 with the corresponding region of Taq (which lacks βi4). Shaded residues are identical between the two sequences. The secondary structures are indicated directly above (for Eco) and below (for Taq) the sequences; filled rectangles denote α-helices, open rectangles denote β-strands, the dashed lines denote disordered regions. The number scale above the Eco secondary structure corresponds to the Eco β subunit sequence. Above the number scale, black lines denote the sequence regions common to all bacterial RNAPs . The yellow and orange lines denote the two BBM2 motifs . The extent of the common β2 domain (thick cyan line) and the lineage-specific insert βi4 (thick green line) is indicated at the top. (B) Ribbon diagram of Eco β2-βi4 (β2 domain, cyan; βi4, green). A disordered loop (Eco β 161–169) is denoted by small spheres. The view corresponds to the reference view of Taq core RNAP (lower left, β-side view), shown as a backbone worm and color-coded as follows: αI, αII, ω, gray; β', light pink; β, light cyan, except the β2 domain is colored cyan and labeled. (C) Ribbon diagram of Eco βi4 (same view as B). The tandem BBM2 motifs predicted by Iyer et al. are color-coded as in (A) (BBM2a, yellow; BBM2b, orange).
Figure 3. Sequence and structure of Eco RNAP βflap-βi9.
(A) Sequence alignment comparing the sequence context of Eco RNAP βi9 with the corresponding region of Taq (which lacks βi9). Shaded residues are identical between the two sequences. The secondary structure for Eco is indicated directly above the sequence; filled rectangles denote α-helices, open rectangles denote β-strands. The number scale above the Eco secondary structure corresponds to the Eco β subunit sequence. Above the number scale, black lines denote the sequence regions common to all bacterial RNAPs . Gaps in the βi9 sequence with numbers above denote the location and residue length of insertions in an alignment of 307 non-redundant βi9 sequences (see Supporting Information). The extent of the common βflap domain (thick cyan line) and the lineage-specific insert βi9 (thick orange line) is indicated at the top. (B) Two orthogonal views of Eco βflap-βi9 (βflap, cyan; βi9, orange). The views correspond to the reference views of Taq core RNAP (left, bottom view; right, front view), shown as a backbone worm and color-coded as follows: αI, αII, ω, gray; β', light pink; β, light cyan, except the βflap domain is colored cyan and labeled.
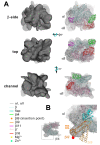
Figure 4. Fitting into cryo-EM densities to generate a molecular model of Eco RNAP.
(A) Three views of the spEM density map and corresponding fit of the Eco RNAP homology model (excluding ω, the C-terminal 41 residues of β', and βi9). For each view (β-side, top, and channel views), the left image shows the spEM density map (grey surface, contoured at 2.5 σ), and the right image shows the spEM density map (grey transparent surface) with the fitted Eco RNAP homology model superimposed. The Eco RNAP homology model is shown as a backbone worm, color-coded as shown in the key (lower left). (B) View of the hEM density map and corresponding fit of the Taq core RNAP crystal structure . The small view (left, which corresponds roughly to the bottom view) shows the entire structure (weak density due to βi4 is noted). The boxed region is magnified on the right, where the Eco βflap-βi9 structure (βflap, cyan; βi9, light orange) is superimposed via the flap domain (excluding the flap-tip). The resulting position of βi9 (light orange) was adjusted to fit into the hEM density (βi9', orange). The red dot denotes the position of a positive difference peak from a hEM reconstruction of a mutant RNAP harboring a 234-residue insertion in βi9 between residues 998 and 999 .
Figure 5. Three views (channel, front, and top) of the Eco RNAP TEC model.
In each view, the RNAP is shown as a molecular surface, and the nucleic acids are shown as phosphate backbone worms (DNA template strand, dark green; DNA nontemplate strand, light green; RNA transcript, gold). Channel view (left): The RNAP is color coded as follows: αI, αII, ω, grey; β, cyan, except βi4 is green, βi9 is orange, and βi11 is magenta; β', pink, except β'i6 is red. The positions of two paf mutants (βR368 and βP372) , are colored blue. β'T1068 (within β'i6), which is phosphorylated by bacteriophage T7 Gp0.7 , is shown in yellow. The thick black arrow points in the downstream direction. Front view (middle): The RNAP molecular surface is colored according to the solvent-exposed electrostatic surface distribution , scaled from –10 kT (red) to +10 kT (blue). The locations of the paf mutants βR368 and βP372, and β'T1068, are denoted. The upstream DNA (us-DNA) is labeled. Top view (right): The RNAP molecular surface is colored according to the solvent-exposed electrostatic surface distribution , scaled from −10 kT (red) to +10 kT (blue). The locations of highly conserved basic residues in βi4 (βR268, R272, and R275) are denoted. In this view, the nucleic acids are fortuitously hidden from view.
Figure 6. Orientational flexibility of βi9.
Bottom view of the Eco RNAP model. The RNAP is shown as a molecular surface (αI, αII, ω, grey; β, light cyan, except βi4 is green and βi11 is magenta; β', light pink) except for βi9, which is shown as a backbone worm. The modeled position of βi9 (see Figure 4B) is colored orange. Selected alternative orientations accessible to βi9 are colored light orange. The potential reach of βi9 maps out roughly a hemisphere with a radius of 65 Å.
Figure 7. Sequence and structural context of Eco RNAP βi11 and Taq βi12.
(A) Sequence alignment comparing the sequence context of Eco RNAP βi11 with the corresponding region of Taq (which lacks βi11 but harbors βi12) . Shaded residues are identical between the two sequences. The experimentally determined secondary structure for Taq is indicated directly below the sequence; filled rectangles denote α-helices, open rectangles denote β-strands. The number scale above the Eco secondary structure corresponds to the Eco β subunit sequence. Above the number scale, black lines denote the sequence regions common to all bacterial RNAPs . The extent of Eco βi11 and Taq βi12 are denoted by the thick magenta line (above) and the thick blue line (below). (B) A portion of the spEM map (contoured at 2.5 σ) is shown (transparent grey surface) with the superimposed Taq core RNAP structure (left, with βi12 colored blue) and the fitted Eco RNAP model (right, with βi11 colored magenta). The view corresponds roughly to the reference view of the Eco RNAP model (top view), shown as a backbone worm and color-coded as follows: αI, αII, ω, gray; β', light pink, except β'i6 is red; β, light cyan, except βi4 is green, βi9 is orange, and βi11 is magenta.
Figure 8. Structural context of Eco β'i6.
(A) β-side view of the Eco RNAP TEC model. The RNAP is shown as a backbone worm (αI, αII, grey; β, cyan, except βi4 is green, βi9 is orange, βi11 is magenta; β', pink, except β'i6 is red). β'T1068 (within β'i6), which is phosphorylated by bacteriophage T7 Gp0.7 , is shown as yellow CPK atoms. The nucleic acids are shown as phosphate backbone worms (DNA template strand, dark green; DNA nontemplate strand, light green; RNA transcript, gold). The thick black arrow points in the downstream direction. The boxed region is magnified in (B). (B) Magnified view of boxed region from (A). The obscuring portion of the β subunit has been removed to reveal the inside surface of the RNAP active site channel. Color-coding is the same as (A) but the BH, TLH1, TLH2, the β'-jaw, and β'i6 are highlighted. The active-site Mg2+-ion is shown as a yellow sphere.
Similar articles
- Insights into Escherichia coli RNA polymerase structure from a combination of x-ray and electron crystallography.
Darst SA, Polyakov A, Richter C, Zhang G. Darst SA, et al. J Struct Biol. 1998 Dec 15;124(2-3):115-22. doi: 10.1006/jsbi.1998.4057. J Struct Biol. 1998. PMID: 10049799 - Cryo-EM structure of Escherichia coli σ70 RNA polymerase and promoter DNA complex revealed a role of σ non-conserved region during the open complex formation.
Narayanan A, Vago FS, Li K, Qayyum MZ, Yernool D, Jiang W, Murakami KS. Narayanan A, et al. J Biol Chem. 2018 May 11;293(19):7367-7375. doi: 10.1074/jbc.RA118.002161. Epub 2018 Mar 26. J Biol Chem. 2018. PMID: 29581236 Free PMC article. - Structural basis of transcription arrest by coliphage HK022 Nun in an Escherichia coli RNA polymerase elongation complex.
Kang JY, Olinares PD, Chen J, Campbell EA, Mustaev A, Chait BT, Gottesman ME, Darst SA. Kang JY, et al. Elife. 2017 Mar 20;6:e25478. doi: 10.7554/eLife.25478. Elife. 2017. PMID: 28318486 Free PMC article. - An Introduction to the Structure and Function of the Catalytic Core Enzyme of Escherichia coli RNA Polymerase.
Sutherland C, Murakami KS. Sutherland C, et al. EcoSal Plus. 2018 Aug;8(1):10.1128/ecosalplus.ESP-0004-2018. doi: 10.1128/ecosalplus.ESP-0004-2018. EcoSal Plus. 2018. PMID: 30109846 Free PMC article. Review. - RNA polymerase structure-function: insights into points of transcriptional regulation.
Severinov K. Severinov K. Curr Opin Microbiol. 2000 Apr;3(2):118-25. doi: 10.1016/s1369-5274(00)00062-x. Curr Opin Microbiol. 2000. PMID: 10744988 Review.
Cited by
- Structural biology of bacterial RNA polymerase.
Murakami KS. Murakami KS. Biomolecules. 2015 May 11;5(2):848-64. doi: 10.3390/biom5020848. Biomolecules. 2015. PMID: 25970587 Free PMC article. Review. - Unravelling the means to an end: RNA polymerase II transcription termination.
Kuehner JN, Pearson EL, Moore C. Kuehner JN, et al. Nat Rev Mol Cell Biol. 2011 May;12(5):283-94. doi: 10.1038/nrm3098. Epub 2011 Apr 13. Nat Rev Mol Cell Biol. 2011. PMID: 21487437 Free PMC article. Review. - The role of E. coli Nus-factors in transcription regulation and transcription:translation coupling: From structure to mechanism.
Burmann BM, Rösch P. Burmann BM, et al. Transcription. 2011 May;2(3):130-134. doi: 10.4161/trns.2.3.15671. Transcription. 2011. PMID: 21922055 Free PMC article. - Trigger-helix folding pathway and SI3 mediate catalysis and hairpin-stabilized pausing by Escherichia coli RNA polymerase.
Windgassen TA, Mooney RA, Nayak D, Palangat M, Zhang J, Landick R. Windgassen TA, et al. Nucleic Acids Res. 2014 Nov 10;42(20):12707-21. doi: 10.1093/nar/gku997. Epub 2014 Oct 21. Nucleic Acids Res. 2014. PMID: 25336618 Free PMC article. - Dynamics of GreB-RNA polymerase interaction allow a proofreading accessory protein to patrol for transcription complexes needing rescue.
Tetone LE, Friedman LJ, Osborne ML, Ravi H, Kyzer S, Stumper SK, Mooney RA, Landick R, Gelles J. Tetone LE, et al. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):E1081-E1090. doi: 10.1073/pnas.1616525114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137878 Free PMC article.
References
- Jokerst R. S, Weeks J. R, Zehring W. A, Greenleaf A. L. Analysis of the gene encoding the largest subunit of RNA polymerase II in Drosophila. Mol Gen Genet. 1989;215:266–275. - PubMed
- Iyer L. M, Koonin E. V, Aravind L. Evolution of bacterial RNA polymerase: implications for large-scale bacterial phylogeny, domain accretion, and horizontal gene transfer. Gene. 2004;335:73–88. - PubMed
- Zhang G, Campbell E. A, Minakhin L, Richter C, Severinov K, et al. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell. 1999;98:811–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM073829/GM/NIGMS NIH HHS/United States
- R37 GM038660/GM/NIGMS NIH HHS/United States
- GM053759/GM/NIGMS NIH HHS/United States
- R01 GM067167/GM/NIGMS NIH HHS/United States
- R01 GM053759/GM/NIGMS NIH HHS/United States
- GM038660/GM/NIGMS NIH HHS/United States
- S10 RR022321/RR/NCRR NIH HHS/United States
- P30 EB009998/EB/NIBIB NIH HHS/United States
- R01 GM061898/GM/NIGMS NIH HHS/United States
- 1S10RR022321-01/RR/NCRR NIH HHS/United States
- R01 GM073829/GM/NIGMS NIH HHS/United States
- R01 GM038660/GM/NIGMS NIH HHS/United States
- GM061898/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources