AXL is an essential factor and therapeutic target for metastatic ovarian cancer - PubMed (original) (raw)
AXL is an essential factor and therapeutic target for metastatic ovarian cancer
Erinn B Rankin et al. Cancer Res. 2010.
Abstract
The receptor tyrosine kinase AXL is thought to play a role in metastasis; however, the therapeutic efficacy of an AXL-targeting agent remains largely untested in metastatic disease. In this study, we defined AXL as a therapeutic target for metastatic ovarian cancer. AXL is primarily expressed in metastases and advanced-stage human ovarian tumors but not in normal ovarian epithelium. Genetic inhibition of AXL in human metastatic ovarian tumor cells is sufficient to prevent the initiation of metastatic disease in vivo. Mechanistically, inhibition of AXL signaling in animals with metastatic disease results in decreased invasion and matrix metalloproteinase activity. Most importantly, soluble human AXL receptors that imposed a specific blockade of the GAS6/AXL pathway had a profound inhibitory effect on progression of established metastatic ovarian cancer without normal tissue toxicity. These results offer the first genetic validation of GAS6/AXL targeting as an effective strategy for inhibition of metastatic tumor progression in vivo. Furthermore, this study defines the soluble AXL receptor as a therapeutic candidate agent for treatment of metastatic ovarian cancer, for which current therapies are ineffective.
© 2010 AACR.
Figures
Figure 1. AXL is highly expressed in Type II ovarian tumors and metastases
Representative images of AXL immunohistochemical staining in normal ovarian epithelium (arrow), Type I (low-grade) serous, Type II (high-grade) serous, and omental metastases derived from patients with serous adenocarcinoma. Note that normal and tumor stroma were negative for AXL staining (*). Scale bar represents 20 ums.
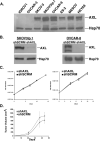
Figure 2. Genetic inactivation of AXL does not affect ovarian tumor cell proliferation in vitro or subcutaneous growth in vivo
A. Western blot analysis of AXL expression in a panel of human ovarian cancer cell lines. B. Western blot analysis of AXL expression in SKOV3ip.1 and OVCAR-8 cells stably transfected with shRNA targeting sequences for scramble control (shSCRM) or AXL (shAXL). Heat shock protein 70 (Hsp70) was used as a protein loading control. C. Cellular growth curves for SKOV3ip.1 (left), and OVCAR-8 (right) shSCRM) and shAXL cells (n = 3). Error bars represent the S.E.M. D. Average tumor volumes of subcutaneous SKOV3ip.1 tumors (n = 4 mice per group) grown over a 48-day time course. Error bars represent the S.E.M.. All full-length blots are presented this figure are in Supplementary Figure 7.
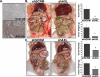
Figure 3. Genetic inactivation of AXL is sufficient to inhibit the initiation of ovarian metastasis
A. AXL immunohistochemical staining (bottom) in peritoneal metastatic lesions from mice injected with shSCRM SKOV3ip.1 cells. B. Representative photographs of mice taken 28 days after injection with shSCRM) and shAXL SKOV3ip.1 cells. Note that the shSCRM injected mice developed numerous metastases in throughout the abdominal cavity (circled). For the shAXL group, the mouse with the greatest tumor burden is shown. Graphs depict the average number of peritoneal metastases >5mm in size per mouse and the average weight of the largest tumor (n = 5 per group). C. Photographs of mice taken 34 days after injection with shSCRM and shAXL OVCAR-8 cells. Note that the shSCRM injected mice developed numerous metastases throughout the abdominal cavity (circled). Large circles represent the original tumor explant inoculation. Graphs depict the average number of peritoneal metastases per mouse and the average tumor weight (n = 8). Asterisks indicate a significant change in tumor burden as determined by the student's t-test (*, p < 0.05).
Figure 4. AXL regulates ovarian tumor cell invasion
Collagen invasion assays of shSCRM and shAXL SKOV3ip.1 and OVCAR-8 cells. Photographs were taken 7 days after plating cells in collagen. Note the invasive phenotype observed in AXL wild type cells (branching) compared to AXL deficient cells (rounded). Graphs show quantification of collagen invasion assays (n = 9).
Figure 5. AXL regulates the PI3K/AKT signaling pathway and MMP expression in ovarian cancer cells
A. Real time PCR analysis of MMP-1, -2, -9, and AXL expression in shAXL and shSCRM ovarian tumor cells. Expression values were normalized to 18S; n = 3. Error bars represent the S.E.M.. B. MMP-2 reporter assay of shSCRM or shAXL SKOV3ip.1 cells (n = 6). C. Gelatin zymography assay for pro- and active-MMP2 activity in conditioned media collected from serum starved SKOV3ip.1 cells. D. Western blot analysis of phospho-AKT at Ser473 (P-AKT), total AKT (AKT), and AXL expression in SKOV3ip.1 cells expressing shRNA sequences targeting shSCRM or shAXL and starved SKOV3ip.1 cells (strve) treated with GAS6 alone or the PI3K inhibitor Ly294002 (Ly). Asterisks indicate a significant increase or decrease in invasion or expression compared to shSCRM as determined by the student's t-test (*, p < 0.01; **, p < 0.001). All full-length blots are presented this figure are in Supplementary Figure 8.
Figure 6. Treatment with soluble AXL receptors inhibits metastatic tumor burden in mice with established metastases
A. Schematic representation of the soluble AXL receptor treatment study. Nude mice were i.p. injected with 1X10e6 SKOV3ip.1 cells. Five days later, the presence of macroscopic lesions was verified in mice. At day 7, mice were injected with adenoviruses expressing the IgG2α-Fc control (Ad-Fc) or soluble AXL receptor (Ad-sAXL). sAXL serum levels were assessed by western blot every 3–4 days following adenoviral injection. Day 28 following tumor cell implantation tumor burden was assessed in all mice. B. Representative photographs of mice treated with AdsAXL or Ad-Fc at day 28. Metastatic lesions are circled. Graphs show the average total tumor number and weight (n = 7). Error bars represent the S.E.M. A statistical difference in tumor number and weight (p=0.01) was observed between Ad-Fc and Ad-sAXL treated mice (*). C. Real time PCR analysis of MMP-2 expression in tumors of mice treated with Ad-Fc or Ad-AXL. All full-length blots are presented in Supplementary Figure 9.
Similar articles
- The GAS6-AXL signaling network is a mesenchymal (Mes) molecular subtype-specific therapeutic target for ovarian cancer.
Antony J, Tan TZ, Kelly Z, Low J, Choolani M, Recchi C, Gabra H, Thiery JP, Huang RY. Antony J, et al. Sci Signal. 2016 Oct 4;9(448):ra97. doi: 10.1126/scisignal.aaf8175. Sci Signal. 2016. PMID: 27703030 - Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies.
Kariolis MS, Miao YR, Diep A, Nash SE, Olcina MM, Jiang D, Jones DS 2nd, Kapur S, Mathews II, Koong AC, Rankin EB, Cochran JR, Giaccia AJ. Kariolis MS, et al. J Clin Invest. 2017 Jan 3;127(1):183-198. doi: 10.1172/JCI85610. Epub 2016 Nov 28. J Clin Invest. 2017. PMID: 27893463 Free PMC article. - Novel Axl-driven signaling pathway and molecular signature characterize high-grade ovarian cancer patients with poor clinical outcome.
Rea K, Pinciroli P, Sensi M, Alciato F, Bisaro B, Lozneanu L, Raspagliesi F, Centritto F, Cabodi S, Defilippi P, Avanzi GC, Canevari S, Tomassetti A. Rea K, et al. Oncotarget. 2015 Oct 13;6(31):30859-75. doi: 10.18632/oncotarget.5087. Oncotarget. 2015. PMID: 26356564 Free PMC article. - AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications.
Zhu C, Wei Y, Wei X. Zhu C, et al. Mol Cancer. 2019 Nov 4;18(1):153. doi: 10.1186/s12943-019-1090-3. Mol Cancer. 2019. PMID: 31684958 Free PMC article. Review. - Therapeutic Targeting of the Gas6/Axl Signaling Pathway in Cancer.
Tanaka M, Siemann DW. Tanaka M, et al. Int J Mol Sci. 2021 Sep 15;22(18):9953. doi: 10.3390/ijms22189953. Int J Mol Sci. 2021. PMID: 34576116 Free PMC article. Review.
Cited by
- High levels of AXL expression in untreated EGFR-mutated non-small cell lung cancer negatively impacts the use of osimertinib.
Yoshimura A, Yamada T, Serizawa M, Uehara H, Tanimura K, Okuma Y, Fukuda A, Watanabe S, Nishioka N, Takeda T, Chihara Y, Takemoto S, Harada T, Hiranuma O, Shirai Y, Shukuya T, Nishiyama A, Goto Y, Shiotsu S, Kunimasa K, Morimoto K, Katayama Y, Suda K, Mitsudomi T, Yano S, Kenmotsu H, Takahashi T, Takayama K. Yoshimura A, et al. Cancer Sci. 2023 Feb;114(2):606-618. doi: 10.1111/cas.15608. Epub 2022 Nov 11. Cancer Sci. 2023. PMID: 36169649 Free PMC article. - Classification of Molecular Subtypes of High-Grade Serous Ovarian Cancer by MALDI-Imaging.
Kassuhn W, Klein O, Darb-Esfahani S, Lammert H, Handzik S, Taube ET, Schmitt WD, Keunecke C, Horst D, Dreher F, George J, Bowtell DD, Dorigo O, Hummel M, Sehouli J, Blüthgen N, Kulbe H, Braicu EI. Kassuhn W, et al. Cancers (Basel). 2021 Mar 25;13(7):1512. doi: 10.3390/cancers13071512. Cancers (Basel). 2021. PMID: 33806030 Free PMC article. - Inhibition of AXL and VEGF-A Has Improved Therapeutic Efficacy in Uterine Serous Cancer.
Toboni MD, Lomonosova E, Bruce SF, Tankou JI, Mullen MM, Schab A, Oplt A, Noia H, Wilke D, Kuroki LM, Hagemann AR, McCourt CK, Thaker PH, Powell MA, Khabele D, Mutch DG, Fuh KC. Toboni MD, et al. Cancers (Basel). 2021 Nov 23;13(23):5877. doi: 10.3390/cancers13235877. Cancers (Basel). 2021. PMID: 34884986 Free PMC article. - Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy.
Leconet W, Larbouret C, Chardès T, Thomas G, Neiveyans M, Busson M, Jarlier M, Radosevic-Robin N, Pugnière M, Bernex F, Penault-Llorca F, Pasquet JM, Pèlegrin A, Robert B. Leconet W, et al. Oncogene. 2014 Nov 20;33(47):5405-14. doi: 10.1038/onc.2013.487. Epub 2013 Nov 18. Oncogene. 2014. PMID: 24240689 Free PMC article. - Evaluation of the Role of AXL in Fusion-positive Pediatric Rhabdomyosarcoma Identifies the Small-molecule Inhibitor Bemcentinib (BGB324) as Potent Chemosensitizer.
Danielli SG, Wurth J, Morice S, Kisele S, Surdez D, Delattre O, Bode PK, Wachtel M, Schäfer BW. Danielli SG, et al. Mol Cancer Ther. 2024 Jun 4;23(6):864-876. doi: 10.1158/1535-7163.MCT-23-0285. Mol Cancer Ther. 2024. PMID: 38471796 Free PMC article.
References
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. - PubMed
- Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7:925–34. - PubMed
- Shan W, Liu J. Inflammation: a hidden path to breaking the spell of ovarian cancer. Cell Cycle. 2009;8:3107–11. - PubMed
- Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007;7:737–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009302/CA/NCI NIH HHS/United States
- P01 CA067166/CA/NCI NIH HHS/United States
- R01 CA116685/CA/NCI NIH HHS/United States
- T32 CA009151/CA/NCI NIH HHS/United States
- CA67166/CA/NCI NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01 DK085527/DK/NIDDK NIH HHS/United States
- F32 CA124082/CA/NCI NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- T32 CA09151/CA/NCI NIH HHS/United States
- CA116685/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous