ARFGAP2 and ARFGAP3 are essential for COPI coat assembly on the Golgi membrane of living cells - PubMed (original) (raw)
ARFGAP2 and ARFGAP3 are essential for COPI coat assembly on the Golgi membrane of living cells
Fredrik Kartberg et al. J Biol Chem. 2010.
Abstract
Coat protein complex I (COPI) vesicles play a central role in the recycling of proteins in the early secretory pathway and transport of proteins within the Golgi stack. Vesicle formation is initiated by the exchange of GDP for GTP on ARF1 (ADP-ribosylation factor 1), which, in turn, recruits the coat protein coatomer to the membrane for selection of cargo and membrane deformation. ARFGAP1 (ARF1 GTPase-activating protein 1) regulates the dynamic cycling of ARF1 on the membrane that results in both cargo concentration and uncoating for the generation of a fusion-competent vesicle. Two human orthologues of the yeast ARFGAP Glo3p, termed ARFGAP2 and ARFGAP3, have been demonstrated to be present on COPI vesicles generated in vitro in the presence of guanosine 5'-3-O-(thio)triphosphate. Here, we investigate the function of these two proteins in living cells and compare it with that of ARFGAP1. We find that ARFGAP2 and ARFGAP3 follow the dynamic behavior of coatomer upon stimulation of vesicle budding in vivo more closely than does ARFGAP1. Electron microscopy of ARFGAP2 and ARFGAP3 knockdowns indicated Golgi unstacking and cisternal shortening similarly to conditions where vesicle uncoating was blocked. Furthermore, the knockdown of both ARFGAP2 and ARFGAP3 prevents proper assembly of the COPI coat lattice for which ARFGAP1 does not seem to play a major role. This suggests that ARFGAP2 and ARFGAP3 are key components of the COPI coat lattice and are necessary for proper vesicle formation.
Figures
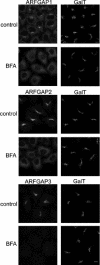
FIGURE 1.
The Golgi localization of the ARFGAPs is dependent on ARFGTP. HeLa wild-type cells were treated with BFA (5 μg/ml) for 1 min and stained with antibodies toward ARFGAP1–3 (left). To verify that this treatment did not affect Golgi structure, cells were co-stained with antibody against β1,4-galactosyltransferase I (GalT; right). Scale bar, 10 μm.
FIGURE 2.
The addition of AlF induces vesicle budding from the Golgi. Micrographs of HeLa WT control cells (A) have typical Golgi stacks. The addition of AlF for 10 min (B) causes massive accumulation of vesicles. Scale bar, 1 μm.
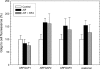
FIGURE 3.
AlF-induced association of ARFGAPs with the Golgi membrane. HeLa cells (n = 25) treated with AlF and BFA were stained with antibodies toward the ARFGAPs and coatomer. Quantification of the Golgi/cell ratio reveals Golgi accumulation of ARFGAP2, ARFGAP3, and coatomer upon the addition of AlF ± S.D. (error bars).
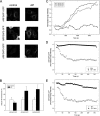
FIGURE 4.
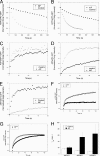
AlF treatment promotes association of the Glo3-type ARFGAP-GFPs with the Golgi membrane. A, HeLa cells expressing GFP-tagged versions of ARFGAP1–3 (left) were treated with AlF (right) and imaged. The addition of AlF increases the association of ARFGAP2-GFP and ARFGAP3-GFP with the Golgi but does not increase the levels of ARFGAP1-GFP. Scale bar, 10 μm. B, Golgi-to-cell fluorescence was quantified on cells (n = 25) expressing the ARFGAPs ± S.D. (error bars). The increase of Golgi association is around 75% for ARFGAP2-GFP and ARFGAP3-GFP, whereas ARFGAP1-GFP decreases about 30% (p < 0.05). C, cells expressing GFP-tagged versions of the ARFGAPs were treated with AlF, and the Golgi intensity was continuously monitored. During a period of 30 min, recruitment of ARFGAP2-GFP (circles) and ARFGAP3-GFP (squares) occurs, whereas ARFGAP1-GFP (triangles) levels are stable. Pretreatment of cells expressing ARFGAP2-GFP (D) or ARFGAP3-GFP (E) with AlF (black circles) protects against BFA-induced redistribution of the Golgi pools.
FIGURE 5.
Golgi association of ARFGAP2 and ARFGAP3 is promoted by ARF1-GTP. A, cells expressing ARF1WT or ARF1Q71L were treated with BFA (5 μg/ml) and stained with antibodies against ARFGAP2 (top) or ARFGAP3 (bottom). Scale bar, 10 μm. B, quantification of this effect. The proportion of cells (percentage of control) exhibiting typical Golgi staining of the ARFGAPs after expression of ARF1Q71L demonstrates the protective effect of this expression.
FIGURE 6.
Dynamic association of ARFGAP2 and ARFGAP3 with the Golgi membrane. Repetitive photobleaching of the cytoplasm causes the loss of the entire pool of fluorescence of ARFGAP2-GFP (A) and ARFGAP3-GFP (white circles) (B), demonstrating the dynamic association of these proteins with the Golgi membrane. AlF treatment (A and B) impairs loss of these proteins (black circles). C–G, recovery of Golgi fluorescence after a single bleach event in the absence (white circles) and presence of AlF (black circles) for ARFGAP1-GFP (C), ARFGAP2-GFP (D), ARFGAP3-GFP (E), ϵ-COP-GFP (F), and ARF1-GFP (G). H, average (n = 12 cells) _t_½ for ARFGAPs treated or not treated with AlF ± S.E. (error bars). The addition of AlF prolongs ARFGAP2-GFP and ARFGAP3-GFP association with Golgi membranes similarly to its effect on ϵ-COP-GFP.
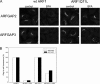
FIGURE 7.
Electron microscopy of cells transfected with RNAiMock and RNAiARFGAP2 and RNAiARFGAP3. Thin plastic embedded sections (60 nm thick) of HeLa cells were examined at the ultrastructural level. Knockdown of ARFGAP2 and ARFGAP3 caused significant structural impairment of the Golgi stacks. Top left, cells transfected with RNAiMock have aligned stacks that are part of the Golgi ribbon. Top right and bottom left, cells transfected with RNAiARFGAP2 and RNAiARFGAP3 show Golgi regions composed of fewer cisternae and stacked structures. Bottom right, quantitation of number of cisternae and stacked cisternae as compared with control (Mock). Error bars, S.E.
FIGURE 8.
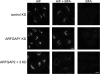
The ARFGAP2 and ARFGAP3 pair is essential for generation of the coat lattice. Cells treated with siRNA against the ARFGAPs or combinations thereof were treated with AlF or BFA or double-treated with AlF and BFA. Treated cells were stained for coatomer. Cells treated with control or siRNA against ARFGAP1 demonstrate BFA resistance after AlF treatment. After double knockdown (KD) of ARFGAP2 and ARFGAP3, coatomer becomes sensitive to BFA with redistribution of coatomer to the cytosol. Scale bar, 11 μm.
FIGURE 9.
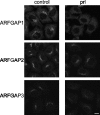
The effect of propranolol treatment on Golgi association of the ARFGAPs. Cells treated with propranolol (prl; 300 μ
m
for 3 min) were stained with antibodies for ARFGAP1 to -3. ARFGAP1 is highly sensitive to propranolol exposure. ARFGAP2 retains its Golgi localization after propranolol treatment, whereas ARFGAP3 has an intermediate sensitivity. Scale bar, 10 μm.
Similar articles
- Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking.
Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brügger B, Wieland F. Weimer C, et al. J Cell Biol. 2008 Nov 17;183(4):725-35. doi: 10.1083/jcb.200806140. J Cell Biol. 2008. PMID: 19015319 Free PMC article. - Two human ARFGAPs associated with COP-I-coated vesicles.
Frigerio G, Grimsey N, Dale M, Majoul I, Duden R. Frigerio G, et al. Traffic. 2007 Nov;8(11):1644-55. doi: 10.1111/j.1600-0854.2007.00631.x. Epub 2007 Sep 29. Traffic. 2007. PMID: 17760859 Free PMC article. - ArfGAP1 dynamics and its role in COPI coat assembly on Golgi membranes of living cells.
Liu W, Duden R, Phair RD, Lippincott-Schwartz J. Liu W, et al. J Cell Biol. 2005 Mar 28;168(7):1053-63. doi: 10.1083/jcb.200410142. J Cell Biol. 2005. PMID: 15795316 Free PMC article. - The COPI system: molecular mechanisms and function.
Beck R, Rawet M, Wieland FT, Cassel D. Beck R, et al. FEBS Lett. 2009 Sep 3;583(17):2701-9. doi: 10.1016/j.febslet.2009.07.032. Epub 2009 Jul 22. FEBS Lett. 2009. PMID: 19631211 Review. - The structure of COPI vesicles and regulation of vesicle turnover.
Taylor RJ, Tagiltsev G, Briggs JAG. Taylor RJ, et al. FEBS Lett. 2023 Mar;597(6):819-835. doi: 10.1002/1873-3468.14560. Epub 2022 Dec 30. FEBS Lett. 2023. PMID: 36513395 Review.
Cited by
- ArfGAP3 is a component of the photoreceptor synaptic ribbon complex and forms an NAD(H)-regulated, redox-sensitive complex with RIBEYE that is important for endocytosis.
Dembla M, Wahl S, Katiyar R, Schmitz F. Dembla M, et al. J Neurosci. 2014 Apr 9;34(15):5245-60. doi: 10.1523/JNEUROSCI.3837-13.2014. J Neurosci. 2014. PMID: 24719103 Free PMC article. - ArfGAP3 regulates the transport of cation-independent mannose 6-phosphate receptor in the post-Golgi compartment.
Shiba Y, Kametaka S, Waguri S, Presley JF, Randazzo PA. Shiba Y, et al. Curr Biol. 2013 Oct 7;23(19):1945-51. doi: 10.1016/j.cub.2013.07.087. Epub 2013 Sep 26. Curr Biol. 2013. PMID: 24076238 Free PMC article. - Integration of genome-wide association and extant brain expression QTL identifies candidate genes influencing prepulse inhibition in inbred F1 mice.
Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Palmer AA. Sittig LJ, et al. Genes Brain Behav. 2016 Feb;15(2):260-70. doi: 10.1111/gbb.12262. Epub 2016 Jan 8. Genes Brain Behav. 2016. PMID: 26482417 Free PMC article. - Recruitment of Arf1-GDP to Golgi by Glo3p-type ArfGAPs is crucial for golgi maintenance and plant growth.
Min MK, Jang M, Lee M, Lee J, Song K, Lee Y, Choi KY, Robinson DG, Hwang I. Min MK, et al. Plant Physiol. 2013 Feb;161(2):676-91. doi: 10.1104/pp.112.209148. Epub 2012 Dec 24. Plant Physiol. 2013. PMID: 23266962 Free PMC article. - ACBD3 modulates KDEL receptor interaction with PKA for its trafficking via tubulovesicular carrier.
Yue X, Qian Y, Zhu L, Gim B, Bao M, Jia J, Jing S, Wang Y, Tan C, Bottanelli F, Ziltener P, Choi S, Hao P, Lee I. Yue X, et al. BMC Biol. 2021 Sep 7;19(1):194. doi: 10.1186/s12915-021-01137-7. BMC Biol. 2021. PMID: 34493279 Free PMC article.
References
- Bonifacino J. S., Glick B. S. (2004) Cell 116, 153–166 - PubMed
- Elsner M., Hashimoto H., Nilsson T. (2003) Mol. Membr. Biol. 20, 221–229 - PubMed
- Rabouille C., Klumperman J. (2005) Nat. Rev. Mol. Cell Biol. 6, 812–817 - PubMed
- D'Souza-Schorey C., Chavrier P. (2006) Nat. Rev. Mol. Cell Biol. 7, 347–358 - PubMed
- Casanova J. E. (2007) Traffic 8, 1476–1485 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials