Cholinergic modulation of neuronal excitability in the accessory olfactory bulb - PubMed (original) (raw)
Comparative Study
. 2010 Dec;104(6):2963-74.
doi: 10.1152/jn.00446.2010. Epub 2010 Sep 22.
Affiliations
- PMID: 20861438
- PMCID: PMC3007668
- DOI: 10.1152/jn.00446.2010
Comparative Study
Cholinergic modulation of neuronal excitability in the accessory olfactory bulb
Richard S Smith et al. J Neurophysiol. 2010 Dec.
Abstract
The accessory olfactory bulb (AOB), the first relay of chemosensory information in the Vomeronasal system, receives extensive cholinergic innervation from the basal forebrain. Cholinergic modulation of neuronal activity in the olfactory bulb has been hypothesized to play an important role in olfactory processing; however, little is known about the cellular actions of acetylcholine (ACh) within the AOB. Here using in vitro slice preparation, we show that muscarinic acetylcholine receptor (mAChR) activation increases neuronal excitability of granule and mitral/tufted cells (GCs and MCs) in the AOB. Activation of mAChRs increased excitability of GCs by three distinct mechanisms: induction of a long-lasting depolarization, activation of a slow afterdepolarization (sADP), and an increase in excitatory glutamatergic input due to MC depolarization. The depolarization and sADP were elicited by the selective agonist 4-[[[(3-chlorophenyl)amino]carbonyl]oxy]-N,N,N-trimethyl-2-butyn-1-aminium chloride (100 μM) and blocked by low concentrations of pirenzepine (300 nM), indicating that they result from activation of M1-like mAChRs. In contrast, cholinergic stimulation increased the excitability of MCs via recruitment of nicotinic AChRs (nAChRs) and M1-like mAChRs. Submaximal activation of these receptors, however, decreased the excitability of MCs. Surprisingly, we found that unlike GCs in the main olfactory bulb, GCs in the AOB are excited by mAChR activation in young postnatal neurons, suggesting marked differences in cholinergic regulation of development between these two regions of the olfactory bulb.
Figures
Fig. 1.
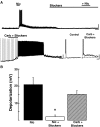
Muscarinic acetylcholine receptor (mAChR) agonists excite granule cells (GCs). A, left: bath application of the nonselective muscarinic agonist oxotremorine (Oxo, 30 μM, 2 min) produced a robust membrane depolarization and sustained firing of action potentials. Right: in addition to membrane depolarization, Oxo (30 μM, 3 min) induced the appearance of a slow afterdepolarization (sADP) following a stimulus-induced train of action potentials (20 pA, 500 ms, right trace). In control the action potentials are followed by a small AHP (↓, see text). B, left trace: low concentration of the M1 mAChR antagonist pirenzepine (Pir, 300 nM) greatly reduced the Oxo-induced depolarization and sADP (right traces). Responses in A and B are from the same cell, the calibration bar is 20 mV and 3 min (traces on the left) and 10 mV and 2 s (traces on the right). The resting membrane potential (RMP) is −65 mV. C: the M1 mAChR agonist 4-[[[(3-chlorophenyl)amino]carbonyl]oxy]-_N,N,N_-trimethyl-2-butyn-1-aminium chloride (MCN-A-343; 100 μM, 3 min) mimics the Oxo-induced depolarization and sADP. Right: superimposed traces showing the stimulus-induced sADP obtained in control and MCN-A-343 from the cell in the middle panel. The calibration bar is 20 mV and 3 min (left traces) and 10 mV and 2 s (right traces); the RMP is −61 mV (left) and −64 mV (right). D: summary of the pharmacological profile of the excitatory muscarinic response in GCs. Pir (□) significantly reduced the Oxo-induced depolarization (■) and sADP (depolarization, top, *P < 0.0005; sADP, bottom, *P < 0.0002). Both excitatory effects were mimicked by MCN-A-343 (▨).
Fig. 2.
Muscarinic but not nicotinic acetylcholine receptor activation directly excites GCs. A, top: bath application of nicotine (Nic, 300 μM, 1 min) produced a robust depolarization that elicited firing of action potentials and increase in excitatory synaptic activity. Application of a mixture of glutamate receptor (GluR) blockers including 100 μM LY367385, 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX), and 100 μM
d
-2-amino-5-phosphonopentanoic acid (APV) produced a decrease in the excitatory synaptic activity and greatly reduced the response to nicotine. The calibration bar is 20 mV and 1 min. Bottom: in the presence of the same mixture of GluR blockers, application of carbachol (30 μM, 3 min) produced a robust depolarization and the appearance of a sADP following a stimulus-induced train of action potentials (20 pA, 500 ms, right trace). The calibration bar is 20 mV and 1 min for the left trace and 10 mV and 2 s for the right-hand traces. The RMP is −60 mV (top) and −61 mV (bottom). B: graph bar summarizing the effects of GluR blockers on the excitatory responses to carbachol (30 μM) and nicotine (300 μM). The nicotinic excitatory response (■) is significantly reduced in the presence of the blockers (□, * P < 0.002), while the response to carbachol is not affected (▨; see text).
Fig. 3.
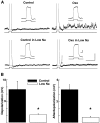
The sADP and depolarization is dependent on extracellular Na. A, top: in the presence of fast synaptic transmission blockers and TTX (1 μM, NBQX 10 μM, and APV 100 μM, see text), Oxo (30 μM) depolarized GCs (not shown) and induced the appearance of sADP (right trace; 5.1 mV in this cell) following a current stimulus (25 pA, 500 ms). Bottom traces: the extracellular Na concentration was reduced to 10 mM with iso-osmolar replacement with NMDG. In low Na, the Oxo-induced sADP following current stimulus (50 pA, 500 ms) and a depolarization (not shown) were almost completely abolished. The dotted line indicates the membrane potential before the depolarizing stimulus, control −64 mV, low Na −67 mV. ↓, the AHP following the current stimulus is not reduced in the low-Na solution (bottom right trace, see text). The calibration bar is 2 s and 10 mV, and 200 ms and 10 mV for the inset. B: graph bar summarizing the effects of low extracellular Na concentration (□) on the depolarization and sADP elicited by Oxo (30 μM); both the depolarization and sADP are significantly reduced in low Na (* P < 0.05, see text).
Fig. 4.
Excitatory muscarinic responses in GCs are present from early postnatal days. A: GCs recorded in slices from postnatal day 6 (P-6, left trace) exhibit a robust depolarization and stimulus-induced sADP (inset) in the presence of Oxo (30 μM). This response is qualitatively similar to the excitatory muscarinic response in adult mice (P-60, right trace). The RMP of both cells is –67 mV; calibration bar is 20 mV and 1 min and 10 mV and 1 s for the inset. B: bar graph showing the average depolarization in postnatal, age-grouped, cells. No significant difference is observed in the degree of depolarization induced by Oxo (30 μM) in these different groups. C: the muscarinic depolarization response in the young mice is insensitive to blockers of excitatory fast synaptic transmission (10 μM NBQX, and 100 μM APV, P < 0.06), but it was greatly reduced by the selective M1 mAChR antagonist Pir (300 nM, P < 0.01).
Fig. 5.
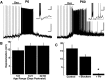
M1-mAChR activation produces an excitatory response in mitral and tufted cells (MCs). A: MCs are depolarized by Oxo (30 μM; 3 min, left), but a train of stimulus-induced action potentials is not followed by a sADP (stimulus: 75 pA, 500 ms, right traces). Compared with GCs, the depolarization elicited by Oxo in MCs has a faster onset (<45 s), but it similarly lasted several minutes (>10 min, see Fig. 1). The RMP in this cell is –62 mV; the calibration bar is 20 mV and 1 min (left) and 10 mV and 1 s (right). B: the excitatory effect of Oxo is mimicked by the selective M1 mAChR agonist MCN-A-343 (100 μM, 2 min, left) and greatly reduced by Pir (300 nM, right). The RMP in these cells is –62 and –66 mV, respectively. C: Oxo (30 μM) still produced a robust depolarization in the presence of blockers of fast excitatory and inhibitory synaptic transmission (100 μM APV, 10 μM CNQX, 5 μM GABAzine). In the presence of blockers, the sADP was still present. D: bar graph summarizing the effects of selective mAChR agonist and antagonists in MCs. The depolarizing response of Oxo (30 μM, ■) was significantly decreased in the presence of Pir (300 nM, □, P < 0.02) and mimicked by MCN-A-343 (100 μM, ▨).
Fig. 6.
Nicotinic AChR activation excites MCs. A: bath application of Nic (30 μM, 1 min) produced a fast-onset depolarization in MCs (<20 s). The membrane potential in this cell is −66 mV. Calibration bar is 20 mV and 2 min. B, top: voltage-clamp recordings showing nAChR activated inward currents in presence of selective agonists and antagonists in the same cell. Nic (10 μM) produced a fast onset inward current (−153 pA), Cho (100 μM) failed to produce an inward current, while Cyt (10 μM) produced a larger response than Nic (−292 pA). The response to Nic was not significantly reduced in the presence of the a7-containing nAChR antagonist MLA (10 nM, −108 pA). Bottom trace: in a different cell, the response to Nic (30 μM, −231 pA) was completely abolished in the presence of MM (30 μM). For both cells, the calibration bar is 100 pA and 1 min. C: sensitivity of the nicotinic response to selective antagonists; the response to Nic was 103 ± 5% in MLA 10 nM, in Blockers (NBQX, APV, BMI and TTX) 60 ± 8%, 34 ± 22% in dihydro-β-erythroidine hydrobromide (DHBE) and 2 ± 2% in mecamylamine hydrochloride (MM, see text, *P < 0.05, ** P < 0.02). D: the inward current produced by Nic (30 μM) showed a strong inward rectification; I-V graph shows the average normalized current at −60 mV in 3 cells. In all cells, the holding potential is −60 mV.
Fig. 7.
Submaximal activation of nicotinic and muscarinic receptors decreases the output from MCs. A, top: a low concentration of Nic (3 μM) reduced the firing rate in this MC. In the same cell, the GABA receptor antagonist GABAzine (5 μM) blocked the inhibitory response produced by Nic and only a slight excitatory remained. Bottom traces: low concentrations of Oxo (3 μM) decreased the firing rate in this MC and GABAzine (5 μM) also reduced this inhibitory effect. Cells were manually clamped at −60 mV; the calibration bar in is 0.5 s and 20 mV. B: graph bar summarizing the effects of submaximal concentrations of Oxo (3 μM) and Nic (3 μM) in the firing frequency of MCs (see text, ■, *P < 0.002). Both Nic and Oxo significantly reduced the frequency of firing. In the presence of GABAzine (5 μM, □), the inhibitory effects of these agonists were greatly diminished leaving a slight excitatory effect.
Similar articles
- Differential Muscarinic Modulation in the Olfactory Bulb.
Smith RS, Hu R, DeSouza A, Eberly CL, Krahe K, Chan W, Araneda RC. Smith RS, et al. J Neurosci. 2015 Jul 29;35(30):10773-85. doi: 10.1523/JNEUROSCI.0099-15.2015. J Neurosci. 2015. PMID: 26224860 Free PMC article. - Excitatory actions of noradrenaline and metabotropic glutamate receptor activation in granule cells of the accessory olfactory bulb.
Smith RS, Weitz CJ, Araneda RC. Smith RS, et al. J Neurophysiol. 2009 Aug;102(2):1103-14. doi: 10.1152/jn.91093.2008. Epub 2009 May 27. J Neurophysiol. 2009. PMID: 19474170 Free PMC article. - Multiple and opposing roles of cholinergic transmission in the main olfactory bulb.
Castillo PE, Carleton A, Vincent JD, Lledo PM. Castillo PE, et al. J Neurosci. 1999 Nov 1;19(21):9180-91. doi: 10.1523/JNEUROSCI.19-21-09180.1999. J Neurosci. 1999. PMID: 10531421 Free PMC article. - Developmental and aging aspects of the cholinergic innervation of the olfactory bulb.
Durand M, Coronas V, Jourdan F, Quirion R. Durand M, et al. Int J Dev Neurosci. 1998 Nov-Dec;16(7-8):777-85. doi: 10.1016/s0736-5748(98)00087-2. Int J Dev Neurosci. 1998. PMID: 10198824 Review. - Influence of age on nicotinic cholinergic regulation of blood flow in rat's olfactory bulb and neocortex.
Uchida S, Kagitani F. Uchida S, et al. J Physiol Sci. 2024 Mar 15;74(1):18. doi: 10.1186/s12576-024-00913-8. J Physiol Sci. 2024. PMID: 38491428 Free PMC article. Review.
Cited by
- A two-layer biophysical model of cholinergic neuromodulation in olfactory bulb.
Li G, Cleland TA. Li G, et al. J Neurosci. 2013 Feb 13;33(7):3037-58. doi: 10.1523/JNEUROSCI.2831-12.2013. J Neurosci. 2013. PMID: 23407960 Free PMC article. - Pharmacological manipulation of the olfactory bulb modulates beta oscillations: testing model predictions.
Osinski BL, Kim A, Xiao W, Mehta NM, Kay LM. Osinski BL, et al. J Neurophysiol. 2018 Sep 1;120(3):1090-1106. doi: 10.1152/jn.00090.2018. Epub 2018 May 30. J Neurophysiol. 2018. PMID: 29847235 Free PMC article. - Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb.
Rothermel M, Carey RM, Puche A, Shipley MT, Wachowiak M. Rothermel M, et al. J Neurosci. 2014 Mar 26;34(13):4654-64. doi: 10.1523/JNEUROSCI.5026-13.2014. J Neurosci. 2014. PMID: 24672011 Free PMC article. - Paying attention to smell: cholinergic signaling in the olfactory bulb.
D'Souza RD, Vijayaraghavan S. D'Souza RD, et al. Front Synaptic Neurosci. 2014 Sep 25;6:21. doi: 10.3389/fnsyn.2014.00021. eCollection 2014. Front Synaptic Neurosci. 2014. PMID: 25309421 Free PMC article. Review. - Mice Lacking M1 and M3 Muscarinic Acetylcholine Receptors Have Impaired Odor Discrimination and Learning.
Chan W, Singh S, Keshav T, Dewan R, Eberly C, Maurer R, Nunez-Parra A, Araneda RC. Chan W, et al. Front Synaptic Neurosci. 2017 Feb 2;9:4. doi: 10.3389/fnsyn.2017.00004. eCollection 2017. Front Synaptic Neurosci. 2017. PMID: 28210219 Free PMC article.
References
- Brennan PA. The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm Behav 46: 231–240, 2004 - PubMed
- Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol 51: 457–481, 1997 - PubMed
- Brennan PA, Peele PF, Towards an understanding of the pregnancy-blocking urinary chemosignals of mice. Biochem Soc Trans 31: 152–155, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources