Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells - PubMed (original) (raw)
Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells
Rashmi Mishra et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Galectins are unconventionally secreted lectins that participate in the formation of glycoprotein lattices that perform a variety of cell surface functions. Galectins also bind glycosphingolipid headgroups with as yet unclear implications for cellular physiology. We report a specific interaction between galectin-9 and the Forssman glycosphingolipid (FGL) that is important for polarizing Madin-Darby canine kidney epithelial cells. Galectin-9 knockdown leads to a severe loss of epithelial polarity that can be rescued by addition of the recombinant protein. The FGL glycan is identified as the surface receptor that cycles galectin-9 to the Golgi apparatus from which the protein is recycled back to the apical surface. Together our results suggest a model wherein such glycosphingolipid-galectin couples form a circuit between the Golgi apparatus and the cell surface that in an epithelial context facilitates the apical sorting of proteins and lipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
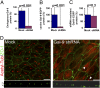
Gal-9 depletion causes morphological and ciliogenesis defects. (A_–_C) A highly sensitive, electrochemiluminescence-based MSD technique was used to quantitate Gal-9 protein levels in retroviral shRNA-mediated knockdown cells. shRNA-susceptible canine and shRNA-resistant human Gal-9–EGFP constructs were stably expressed in MDCK cell lines to test the specificity of shRNA-mediated down-regulation. Samples were normalized to amounts of GAPDH. A one-tail, unpaired t test was used to generate P values. Error bars indicate SD. The data represent two mock-infected and shRNA cell pairs from three independent experimental groups. (D) Acetylated tubulin stains ciliary axoneme and basal body; ZO-1 reveals TJ areas. shRNA cells are enlarged, fusiform, and flat and show complete lack of cilium with occasional staining of the basal body (white arrowheads). (Scale bars: 10 μm.) The x–z view reveals TJs at varying heights.
Fig. 2.
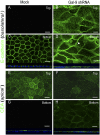
Gal-9 depletion causes mislocalization of protein markers for apical and basolateral polarity. (A, C, E, and G) Mock-infected MDCK cells that were filter-grown for 5 d show E-cadherin localized to the basolateral membrane and CEA localized to the apical membrane. Top, merge of the three 0.5-μm slices farthest from the filter support used to grow the cells. Bottom, merge of three 0.5-μm slices near the filter support (attached surface). (B, D, F, and H) Gal-9 shRNA cells show E-cadherin and CEA expression in all planes, x_–_z views below C–D and G–H respectively. E-cadherin also showed an intracellular punctate staining (white arrowhead in D); (Scale bars: 10 μm.).
Fig. 3.
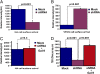
Gal-9 depletion causes transport defects that are rescued with recombinant Gal-9 protein. Adenoviruses expressing the apical cargo protein HA-M2-GFP or basolateral cargo protein VSVG-SP–GFP were harvested for total cell-surface MSD-based transport assays. (A and B) HA surface signal was fourfold lower, and VSV-G surface signal was sixfold higher, after 45 min of cargo release from TGN as compared with the mock-infected cells. (C) shRNA cells were treated with 0.15 μM recombinant Gal-9 for 5 d. The HA transport assay showed surface delivery comparable to mock-infected conditions. Samples in A_–_C are normalized to levels of GFP in lysates used to indicate transfection efficiency. (D) Gal-9–treated shRNA cells showed a recovery of TER comparable to levels in mock-infected cells. The data represent three independent experiments conducted in duplicate. P values were generated from a one-tailed, unpaired t test. Error bars indicate SD.
Fig. 4.
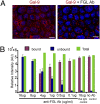
Recombinant Gal-9 rescues ciliogenesis and steady-state expression of apical and basolateral marker proteins. (A) Filter-grown mock-infected and Gal-9–treated shRNA cells were immunostained with anti-acetylated tubulin to visualize the cilium. (Scale bar: 10 μm.) (B and C) The number of cells positive for either a ciliary base or cilia in Gal-9–treated shRNA cells is comparable to the number in mock-infected cells, but the Gal-9–treated shRNA cells show a twofold increase in ciliary length. (D) Mock-, shRNA-, and Gal-9–treated shRNA cells were grown on a filter for 5 d, biotinylated from both sides of the filter, and subjected to MSD-based assay. (E) Mock- and Gal-9–treated shRNA cells were biotinylated from either the apical or the basolateral side and subjected to MSD assay to generate a steady-state profile of relative amounts of CEA and E-cadherin Samples were normalized to amounts of GAPDH detected in cell lysates. The data represent the results of three independent experimental sets conducted in duplicate. ap, apical membrane; AU, arbitrary unit. bl, basolateral membrane. P values are generated from a one-tailed, unpaired t test. Error bars indicate SD.
Fig. 5.
FGL is a receptor for Gal-9 on the apical surface of MDCK cells. (A) Fully polarized MDCK epithelial cells were incubated with varying concentrations of anti-FGL antibody (μg/mL) on ice for 45 min. Biotinylated recombinant Gal-9 (0.01 μM) was allowed to bind for 1 h on ice. Cells were fixed, immunostained with anti-biotin antibody without permeabilizing the cells, and were counterstained with DAPI. (Scale bars: 10 μm.) (B) The unbound fraction was collected, and the bound fraction was stripped from the cellular membrane with a pH drop. Biotin–Gal-9 in each sample was quantitated by MSD assay. Rat IgG was used to negate any nonspecific antibody-mediated effects. Green bars indicate the sum of bound and unbound fractions. The data represent results from three independent experimental sets performed in duplicate. Error bars indicate SD.
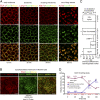
Fig. 6.
Internalization and recycling of recombinant biotin–Gal-9. Biotinylated recombinant Gal-9 (0.01 μM) was bound to the apical membrane on ice, and the internalization to different cellular regions was followed over time. (A_–_C) After 10 min, Gal-9 was found inside the cells and colocalized with the marker of early endosome, EEA1. No significant colocalization was detected with the lysosomal marker Lamp1, but some colocalization was observed with recycling endosome (Rab11) compartments at around 30 min of internalization. Within 60 min, a major fraction of Gal-9 was detected in the TGN compartment, revealed through colocalization with furin-convertase staining. Gal-9 was seen at the base as tubular extensions emanating from the TGN (indicated by white arrowheads in the Furin/Gal-9/Merge, Bottom Right). After 2 h, biotin–Gal-9 localized primarily with FGL on the apical membrane (
SI Materials and Methods
). (Scale bars in A and B: 10 μm.) (D) Time-course analysis, using the MSD assay, shows the percentage of biotin–Gal-9 reaching the apical surface and the amount remaining in the cell lysates. Graph shows relative intensity of signal detected at the apical surface and in the cell lysates normalized to the amount of phospholipids in each sample. Individual values for per cent of arrival at each time point are indicated. Error bars in C and D indicate SD. Details on quantitation are given in
SI Materials and Methods
.
Similar articles
- Galectin-8 regulates targeting of Gp135/podocalyxin and lumen formation at the apical surface of renal epithelial cells.
Lim H, Yu CY, Jou TS. Lim H, et al. FASEB J. 2017 Nov;31(11):4917-4927. doi: 10.1096/fj.201601386R. Epub 2017 Jul 26. FASEB J. 2017. PMID: 28747404 - Sialylation of N-linked glycans mediates apical delivery of endolyn in MDCK cells via a galectin-9-dependent mechanism.
Mo D, Costa SA, Ihrke G, Youker RT, Pastor-Soler N, Hughey RP, Weisz OA. Mo D, et al. Mol Biol Cell. 2012 Sep;23(18):3636-46. doi: 10.1091/mbc.E12-04-0329. Epub 2012 Aug 1. Mol Biol Cell. 2012. PMID: 22855528 Free PMC article. - MAL mediates apical transport of secretory proteins in polarized epithelial Madin-Darby canine kidney cells.
Martín-Belmonte F, Arvan P, Alonso MA. Martín-Belmonte F, et al. J Biol Chem. 2001 Dec 28;276(52):49337-42. doi: 10.1074/jbc.M106882200. Epub 2001 Oct 22. J Biol Chem. 2001. PMID: 11673461 - Recycling of galectin-3 in epithelial cells.
Hönig E, Schneider K, Jacob R. Hönig E, et al. Eur J Cell Biol. 2015 Jul-Sep;94(7-9):309-15. doi: 10.1016/j.ejcb.2015.05.004. Epub 2015 Jun 1. Eur J Cell Biol. 2015. PMID: 26059399 Review. - Proteoglycan synthesis and Golgi organization in polarized epithelial cells.
Dick G, Akslen-Hoel LK, Grøndahl F, Kjos I, Prydz K. Dick G, et al. J Histochem Cytochem. 2012 Dec;60(12):926-35. doi: 10.1369/0022155412461256. Epub 2012 Sep 1. J Histochem Cytochem. 2012. PMID: 22941419 Free PMC article. Review.
Cited by
- Lumenal Galectin-9-Lamp2 interaction regulates lysosome and autophagy to prevent pathogenesis in the intestine and pancreas.
Sudhakar JN, Lu HH, Chiang HY, Suen CS, Hwang MJ, Wu SY, Shen CN, Chang YM, Li FA, Liu FT, Shui JW. Sudhakar JN, et al. Nat Commun. 2020 Aug 27;11(1):4286. doi: 10.1038/s41467-020-18102-7. Nat Commun. 2020. PMID: 32855403 Free PMC article. - Galectin-9 - ligand axis: an emerging therapeutic target for multiple myeloma.
Shil RK, Mohammed NBB, Dimitroff CJ. Shil RK, et al. Front Immunol. 2024 Sep 25;15:1469794. doi: 10.3389/fimmu.2024.1469794. eCollection 2024. Front Immunol. 2024. PMID: 39386209 Free PMC article. Review. - Cell surface galectin-9 expressing Th cells regulate Th17 and Foxp3+ Treg development by galectin-9 secretion.
Oomizu S, Arikawa T, Niki T, Kadowaki T, Ueno M, Nishi N, Yamauchi A, Hattori T, Masaki T, Hirashima M. Oomizu S, et al. PLoS One. 2012;7(11):e48574. doi: 10.1371/journal.pone.0048574. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23144904 Free PMC article. - Membrane Lipids in Epithelial Polarity: Sorting out the PIPs.
Bugda Gwilt K, Thiagarajah JR. Bugda Gwilt K, et al. Front Cell Dev Biol. 2022 May 31;10:893960. doi: 10.3389/fcell.2022.893960. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35712665 Free PMC article. Review. - Novel role for galectin-8 protein as mediator of coagulation factor V endocytosis by megakaryocytes.
Zappelli C, van der Zwaan C, Thijssen-Timmer DC, Mertens K, Meijer AB. Zappelli C, et al. J Biol Chem. 2012 Mar 9;287(11):8327-35. doi: 10.1074/jbc.M111.305151. Epub 2012 Jan 20. J Biol Chem. 2012. PMID: 22267735 Free PMC article.
References
- Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. - PubMed
- Rémillard-Labrosse G, Mihai C, Duron J, Guay G, Lippé R. Protein kinase D-dependent trafficking of the large Herpes simplex virus type 1 capsids from the TGN to plasma membrane. Traffic. 2009;10:1074–1083. - PubMed
- Cooper DN. Galectinomics: Finding themes in complexity. Biochim Biophys Acta. 2002;1572:209–231. - PubMed
- Hughes RC. Galectins in kidney development. Glycoconj J. 2004;19:621–629. - PubMed
- Delacour D, et al. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials