Frontocingulate dysfunction in depression: toward biomarkers of treatment response - PubMed (original) (raw)
Review
Frontocingulate dysfunction in depression: toward biomarkers of treatment response
Diego A Pizzagalli. Neuropsychopharmacology. 2011 Jan.
Abstract
Increased rostral anterior cingulate cortex (rACC) activity has emerged as a promising predictor of treatment response in depression, but neither the reliability of this relationship nor the mechanisms supporting it have been thoroughly investigated. This review takes a three-pronged approach to these issues. First, I present a meta-analysis demonstrating that the relationship between resting rACC activity and treatment response is robust. Second, I propose that the rACC plays a key role in treatment outcome because of its 'hub' position in the default network. Specifically, I hypothesize that elevated resting rACC activity confers better treatment outcomes by fostering adaptive self-referential processing and by helping to recalibrate relationships between the default network and a 'task-positive network' that comprises dorsolateral prefrontal and dorsal cingulate regions implicated in cognitive control. Third, I support this hypothesis by reviewing neuropsychological, electrophysiological, and neuroimaging data on frontocingulate dysfunction in depression. The review ends with a discussion of the limitations of current work and future directions.
Figures
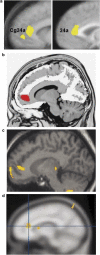
Figure 1
Selected findings implicating the rACC in treatment response in MDD. (a) Mayberg et al (1997): Increased pre-treatment rACC metabolism in responders relative to nonresponders (yellow). This finding was replicated in a larger sample of 25 responders vs 20 nonresponders (Brannan et al, 2000) (see right panel). (b) Pizzagalli et al (2001): Increased pre-treatment resting rACC theta activity in responders relative to nonresponders (red). (c) Holthoff et al (2004): Decreased rACC rCBF with symptom remission (yellow). (d) Davidson et al (2003): Increased BOLD signal in the rACC in response to emotional pictures correlated with lower post-treatment depressive symptoms (yellow). (Modified with permission from Mayberg, 2003 (with permission by Oxford University Press), Pizzagalli et al, 2001 (with permission by American Psychiatric Associations), Holthoff et al, 2004 (with permission by John Wiley and Sons), and Davidson et al, 2003 (with permission by American Psychiatric Associations)).
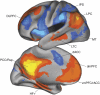
Figure 2
The default network (orange colors) includes regions that deactivate during processing of external stimuli, including the vmPFC/rACC, posterior cingulate (PCC), retrosplenial cortex (Rsp), lateral parietal cortex (LPC), lateral temporal cortex (LTC), dorsal medial PFC (dmPFC), and hippocampal formation (HF+), which includes the entorhinal cortex and surrounding cortex (eg, parahippocampal cortex). The task-positive network (blue color) includes, among others, the DLPFC, dACC, intraparietal sulcus (IPS), and middle temporal (MT) area and becomes activated during tasks requiring cognitive and attentional control. Blue colors: regions that negatively correlate with the default network; red: regions that positively correlate with the default network. (Modified with permission from Buckner et al, 2008; with permission by John Wiley and Sons).
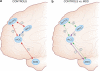
Figure 3
(a) Schematic representation of frontocingulate and frontolimbic interactions associated with adaptive forms of reflective, self-focused processing, as well as adaptive regulation of cognition and emotions. In controls, increased resting rACC activity as well as functional coupling (positive correlations) between the rACC and amygdala (see arrow 1) are observed during resting states (Margulies et al, 2007) and self-referential processing (Schmitz and Johnson, 2006). When confronted with cognitive or affective challenges, healthy controls show increased coupling (positive correlations) between the (1) rACC and DLPFC (arrow 2; Holmes and Pizzagalli, 2008b; Etkin et al, 2006) and (2) DLPFC and dACC (arrow 3; Aizenstein et al, 2009; Fox et al, 2005; Margulies et al, 2007; Schmitz and Johnson, 2006). The interplay among these regions is hypothesized to reduce task-induced rACC activation (arrow 4; Drevets and Raichle, 1998; Fox et al, 2005; Margulies et al, 2007) and downregulate amygdala activation, fostering adaptive regulation of cognition and emotions. (b) Relative to controls, MDD subjects show stronger functional coupling (positive correlations) between the rACC and the amygdala during negative self-referential processing (arrow 1; Yoshimura et al, 2010) as well as reduced structural connectivity between these two regions (arrow 5; Cullen et al, 2010). In addition, relative to controls, MDD subjects show reduced functional connectivity between the (1) rACC and DLPFC (arrow 2; Holmes and Pizzagalli, 2008b; Siegle et al, 2007) and (2) DLPFC and dACC (arrow 3; Aizenstein et al, 2009; Schlösser et al, 2008), but abnormally elevated functional connectivity between the dACC and rACC (arrow 4; Schlösser et al, 2008) during cognitive and/or affective challenges. The dysregulated interplay among these regions is hypothesized to lead to failures to deactivate the rACC and amygdala during affective and cognitive challenges, fostering the emergence of maladaptive forms of rumination, and ultimately treatment nonresponse. Numbers do not reflect chronological unfolding of interactions among brain regions.
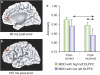
Figure 4
Relative to healthy controls, unmedicated individuals with MDD showed (a) potentiated rACC responses 80 ms after committing an error in a Stroop task, and (b) decreased functional connectivity between rACC activation 80 ms post-error and left DLPFC 472 ms post-error. Among the depressed sample, individuals with the highest left DLPFC activation 472 ms post-error showed more adaptive post-error behavioral adjustments (higher accuracy after errors) relative to MDD participants with the lowest DLPFC recruitment. (Modified with permission from Holmes and Pizzagalli, 2008b. Copyright © 2008 American Medical Association. All rights reserved).
Similar articles
- Pretreatment Rostral Anterior Cingulate Cortex Connectivity With Salience Network Predicts Depression Recovery: Findings From the EMBARC Randomized Clinical Trial.
Whitton AE, Webb CA, Dillon DG, Kayser J, Rutherford A, Goer F, Fava M, McGrath P, Weissman M, Parsey R, Adams P, Trombello JM, Cooper C, Deldin P, Oquendo MA, McInnis MG, Carmody T, Bruder G, Trivedi MH, Pizzagalli DA. Whitton AE, et al. Biol Psychiatry. 2019 May 15;85(10):872-880. doi: 10.1016/j.biopsych.2018.12.007. Epub 2018 Dec 19. Biol Psychiatry. 2019. PMID: 30718038 Free PMC article. Clinical Trial. - Frontal and rostral anterior cingulate (rACC) theta EEG in depression: implications for treatment outcome?
Arns M, Etkin A, Hegerl U, Williams LM, DeBattista C, Palmer DM, Fitzgerald PB, Harris A, deBeuss R, Gordon E. Arns M, et al. Eur Neuropsychopharmacol. 2015 Aug;25(8):1190-200. doi: 10.1016/j.euroneuro.2015.03.007. Epub 2015 Apr 20. Eur Neuropsychopharmacol. 2015. PMID: 25936227 Clinical Trial. - Cognition-Modulated Frontal Activity in Prediction and Augmentation of Antidepressant Efficacy: A Randomized Controlled Pilot Study.
Li CT, Hsieh JC, Huang HH, Chen MH, Juan CH, Tu PC, Lee YC, Wang SJ, Cheng CM, Su TP. Li CT, et al. Cereb Cortex. 2016 Jan;26(1):202-10. doi: 10.1093/cercor/bhu191. Epub 2014 Aug 27. Cereb Cortex. 2016. PMID: 25165064 Clinical Trial. - A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression.
Diener C, Kuehner C, Brusniak W, Ubl B, Wessa M, Flor H. Diener C, et al. Neuroimage. 2012 Jul 2;61(3):677-85. doi: 10.1016/j.neuroimage.2012.04.005. Epub 2012 Apr 12. Neuroimage. 2012. PMID: 22521254 Review. - Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder.
Fonseka TM, MacQueen GM, Kennedy SH. Fonseka TM, et al. J Affect Disord. 2018 Jun;233:21-35. doi: 10.1016/j.jad.2017.10.049. Epub 2017 Oct 31. J Affect Disord. 2018. PMID: 29150145 Review.
Cited by
- Modulation of neural networks and symptom correlated in fibromyalgia: A randomized double-blind multi-group explanatory clinical trial of home-based transcranial direct current stimulation.
Lopes Alves R, Zortea M, Vicuña Serrano P, Laranjeira VDS, Franceschini Tocchetto B, Ramalho L, Fernanda da Silveira Alves C, Brugnera Tomedi R, Pereira de Almeida R, Machado Bruck S, Medeiros L, R S Sanches P, P Silva D Jr, Torres ILS, Fregni F, Caumo W. Lopes Alves R, et al. PLoS One. 2024 Nov 13;19(11):e0288830. doi: 10.1371/journal.pone.0288830. eCollection 2024. PLoS One. 2024. PMID: 39536019 Free PMC article. Clinical Trial. - Safety and efficacy of individual target transcranial magnetic stimulation to stimulate the most negative correlate of DLPFC-pgACC in the treatment of major depressive disorder: study protocol of a double-blind, randomised controlled trial.
Liu N, Zhao N, Tang N, Cai M, Zhang Y, Lv R, Zhang Y, Han T, Meng Y, Zang Y, Wang H. Liu N, et al. BMJ Open. 2024 Nov 7;14(11):e081520. doi: 10.1136/bmjopen-2023-081520. BMJ Open. 2024. PMID: 39515856 Free PMC article. - Unveiling Frequency-Specific Microstate Correlates of Anxiety and Depression Symptoms.
Xue S, Shen X, Zhang D, Sang Z, Long Q, Song S, Wu J. Xue S, et al. Brain Topogr. 2024 Nov 5;38(1):12. doi: 10.1007/s10548-024-01082-y. Brain Topogr. 2024. PMID: 39499403 - Depression and metabolic connectivity: insights into the locus coeruleus, HF-rTMS, and anxiety.
Wu GR, Baeken C. Wu GR, et al. Transl Psychiatry. 2024 Nov 2;14(1):459. doi: 10.1038/s41398-024-03171-9. Transl Psychiatry. 2024. PMID: 39488540 Free PMC article. - Highfield imaging of the subgenual anterior cingulate cortex in uni- and bipolar depression.
Buchholz F, Meffert M, Bazin PL, Trampel R, Turner R, Schönknecht P. Buchholz F, et al. Front Psychiatry. 2024 Oct 11;15:1462919. doi: 10.3389/fpsyt.2024.1462919. eCollection 2024. Front Psychiatry. 2024. PMID: 39465046 Free PMC article.
References
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K.et al (2007HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions Psychiatry Res 155245–256. - PubMed
- Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929–1932. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AT002974/AT/NCCIH NIH HHS/United States
- R21MH078979/MH/NIMH NIH HHS/United States
- R01 MH068376-07/MH/NIMH NIH HHS/United States
- R01 MH068376-01A1/MH/NIMH NIH HHS/United States
- R01 MH068376-06A1/MH/NIMH NIH HHS/United States
- R01 MH068376-03/MH/NIMH NIH HHS/United States
- R21 AT002974-01A1/AT/NCCIH NIH HHS/United States
- R21 MH078979-02/MH/NIMH NIH HHS/United States
- R01 MH068376-08/MH/NIMH NIH HHS/United States
- R01 MH068376-05/MH/NIMH NIH HHS/United States
- R21 MH078979/MH/NIMH NIH HHS/United States
- R01 MH068376/MH/NIMH NIH HHS/United States
- R01MH68376/MH/NIMH NIH HHS/United States
- R21 AT002974-02/AT/NCCIH NIH HHS/United States
- R01 MH068376-02/MH/NIMH NIH HHS/United States
- R21 MH078979-01A2/MH/NIMH NIH HHS/United States
- R01 MH068376-04/MH/NIMH NIH HHS/United States
- R21 MH078979-03/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources