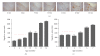Pathological Hallmarks, Clinical Parallels, and Value for Drug Testing in Alzheimer's Disease of the APP[V717I] London Transgenic Mouse Model - PubMed (original) (raw)
Pathological Hallmarks, Clinical Parallels, and Value for Drug Testing in Alzheimer's Disease of the APP[V717I] London Transgenic Mouse Model
An Tanghe et al. Int J Alzheimers Dis. 2010.
Abstract
The APP[V717I] London (APP-Ld) mouse model recapitulates important pathological and clinical hallmarks of Alzheimer's disease (AD) and is therefore a valuable paradigm for evaluating therapeutic candidates. Historically, both the parenchymal and vascular amyloid deposits, and more recently, truncated and pyroglutamate-modified Abeta(3(pE)-42) species, are perceived as important hallmarks of AD-pathology. Late stage symptoms are preceded by robust deficits in orientation and memory that correlate in time with Abeta oligomerization and GSK3β-mediated phosphorylation of endogenous murine Tau, all markers that have gained considerable interest during the last decade. Clinical parallels with AD patients and the value of the APP-Ld transgenic mouse model for preclinical in vivo testing of candidate drugs are discussed.
Figures
Figure 1
Abeta aggregation prior to plaque formation in APP[V717I] (APP-Ld) and APP[V717I] × PS1[A246E] mice. (a) Representative photo collection of anti-Abeta stained sections showing total plaque load in APP-Ld mice of different ages (proprietary anti-Abeta Nanobody, reMYND/Ablynx, Belgium). (b) Aggregated Abeta in APP-Ld mice of different ages, both in preplaque (indicated by the dashed line) and postplaque stages of the Alzheimer pathology (A4-assay, Amorfix Life Sciences Ltd., Mississauga, Canada). The signal of nontransgenic mice was under the S/N cutoff value (data not shown). (c) As in B, for the APP[V717I] × PS1[A246E] model. The signal of nontransgenic mice was under the S/N cutoff value (data not shown).
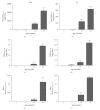
Figure 2
Abeta3(pE)–42 determination in the SDS and FA extracts of APP-Ld brain. Pan-Abeta42 (a, b) and Abeta3(pE)–42 (c,d) concentrations in SDS (a, c) and FA (b, d), as well as the corresponding Abeta3(pE)–42 to pan-Abeta42 ratios (in %) (e, f).
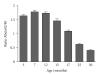
Figure 3
Progression of CSF Abeta42/40 ratios in ageing APP-Ld mice; from the age of 15 months onwards, the ratio of Abeta42/40 in CSF decreases.
Similar articles
- Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.
Dewachter I, van Dorpe J, Spittaels K, Tesseur I, Van Den Haute C, Moechars D, Van Leuven F. Dewachter I, et al. Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review. - Fuzhisan ameliorates Aβ production and tau phosphorylation in hippocampal of 11month old APP/PS1 transgenic mice: A Western blot study.
Zhang ZX, Zhao RP, Wang DS, Wang AN. Zhang ZX, et al. Exp Gerontol. 2016 Nov;84:88-95. doi: 10.1016/j.exger.2016.09.003. Epub 2016 Sep 7. Exp Gerontol. 2016. PMID: 27612601 - Pyroglutamate-modified amyloid-β protein demonstrates similar properties in an Alzheimer's disease familial mutant knock-in mouse and Alzheimer's disease brain.
Wu G, Miller RA, Connolly B, Marcus J, Renger J, Savage MJ. Wu G, et al. Neurodegener Dis. 2014;14(2):53-66. doi: 10.1159/000353634. Epub 2013 Oct 23. Neurodegener Dis. 2014. PMID: 24158021 - Galectin-3 promotes Aβ oligomerization and Aβ toxicity in a mouse model of Alzheimer's disease.
Tao CC, Cheng KM, Ma YL, Hsu WL, Chen YC, Fuh JL, Lee WJ, Chao CC, Lee EHY. Tao CC, et al. Cell Death Differ. 2020 Jan;27(1):192-209. doi: 10.1038/s41418-019-0348-z. Epub 2019 May 24. Cell Death Differ. 2020. PMID: 31127200 Free PMC article. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Meprin β knockout reduces brain Aβ levels and rescues learning and memory impairments in the APP/lon mouse model for Alzheimer's disease.
Marengo L, Armbrust F, Schoenherr C, Storck SE, Schmitt U, Zampar S, Wirths O, Altmeppen H, Glatzel M, Kaether C, Weggen S, Becker-Pauly C, Pietrzik CU. Marengo L, et al. Cell Mol Life Sci. 2022 Mar 2;79(3):168. doi: 10.1007/s00018-022-04205-5. Cell Mol Life Sci. 2022. PMID: 35235058 Free PMC article. - Combined treatment with a BACE inhibitor and anti-Aβ antibody gantenerumab enhances amyloid reduction in APPLondon mice.
Jacobsen H, Ozmen L, Caruso A, Narquizian R, Hilpert H, Jacobsen B, Terwel D, Tanghe A, Bohrmann B. Jacobsen H, et al. J Neurosci. 2014 Aug 27;34(35):11621-30. doi: 10.1523/JNEUROSCI.1405-14.2014. J Neurosci. 2014. PMID: 25164658 Free PMC article. - Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease.
Francis BM, Yang J, Hajderi E, Brown ME, Michalski B, McLaurin J, Fahnestock M, Mount HT. Francis BM, et al. Neuropsychopharmacology. 2012 Jul;37(8):1934-44. doi: 10.1038/npp.2012.40. Epub 2012 Apr 11. Neuropsychopharmacology. 2012. PMID: 22491352 Free PMC article. - Neurodegeneration of the retina in mouse models of Alzheimer's disease: what can we learn from the retina?
Chiu K, Chan TF, Wu A, Leung IY, So KF, Chang RC. Chiu K, et al. Age (Dordr). 2012 Jun;34(3):633-49. doi: 10.1007/s11357-011-9260-2. Epub 2011 May 11. Age (Dordr). 2012. PMID: 21559868 Free PMC article. Review. - Increased CSF Aβ during the very early phase of cerebral Aβ deposition in mouse models.
Maia LF, Kaeser SA, Reichwald J, Lambert M, Obermüller U, Schelle J, Odenthal J, Martus P, Staufenbiel M, Jucker M. Maia LF, et al. EMBO Mol Med. 2015 Jul;7(7):895-903. doi: 10.15252/emmm.201505026. EMBO Mol Med. 2015. PMID: 25978969 Free PMC article.
References
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiology of Aging. 2003;24(8):1063–1070. - PubMed
- Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. Journal of Neurochemistry. 2009;110(4):1129–1134. - PubMed
- Torres-Aleman I. Mouse models of Alzheimer’s dementia: current concepts and new trends. Endocrinology. 2008;149(12):5952–5957. - PubMed
- Jaworski T, Dewachter I, Seymour CM, et al. Alzheimer’s disease: old problem, new views from transgenic and viral models. Biochimica et Biophysica Acta. 2010;1802(10):808–818. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials