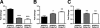CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models - PubMed (original) (raw)
CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models
Sungho Lee et al. Am J Pathol. 2010 Nov.
Abstract
Microglia, the primary immune effector cells in the brain, continually monitor the tissue parenchyma for pathological alterations and become activated in Alzheimer's disease. Loss of signaling between neurons and microglia via deletion of the microglial receptor, CX3CR1, worsens phenotypes in various models of neurodegenerative diseases. In contrast, CX3CR1 deficiency ameliorates pathology in murine stroke models. To examine the role of CX3CR1 in Alzheimer's disease-related β-amyloid pathology, we generated APPPS1 and R1.40 transgenic mouse models of Alzheimer's disease deficient for CX3CR1. Surprisingly, CX3CR1 deficiency resulted in a gene dose-dependent reduction in β-amyloid deposition in both the APPPS1 and R1.40 mouse models of AD. Immunohistochemical analysis revealed reduced staining for CD68, a marker of microglial activation. Furthermore, quantitative immunohistochemical analysis revealed reduced numbers of microglia surrounding β-amyloid deposits in the CX3CR1-deficient APPPS1 animals. The reduced β-amyloid pathology correlated with reduced levels of TNFα and CCL2 mRNAs, but elevated IL1β mRNA levels, suggesting an altered neuroinflammatory milieu. Finally, to account for these seemingly disparate results, both in vitro and in vivo studies provided evidence that CX3CL1/CX3CR1 signaling alters the phagocytic capacity of microglia, including the uptake of Aβ fibrils. Taken together, these results demonstrate that loss of neuron-microglial fractalkine signaling leads to reduced β-amyloid deposition in mouse models of AD that is potentially mediated by altered activation and phagocytic capability of CX3CR1-deficient microglia.
Figures
Figure 1
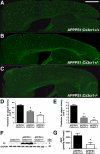
Gene dose-dependent reduction in fibrillar Aβ deposition in the APPPS1 mouse model of AD with CX3CR1 deficiency. Brain sections (30 μm) from APPPS1;Cx3cr1+/+ (A, n = 7), APPPS1;Cx3cr1+/− (B, n = 6), and APPPS1;_Cx3cr1_−/− (C, n = 7) mice at 4 months of age were stained with Thioflavine S. A series of low-power images (Scale bar = 1 mm) were used to reconstruct the cortex and hippocampus from four sections from each animal. As expected based on the published literature, APPPS1;Cx3cr1+/+ animals exhibit abundant fibrillar Aβ deposition throughout the cortex with reduced Aβ deposition in the hippocampus (A). By contrast, age-matched APPPS1;Cx3cr1+/− (B) and APPPS1;_Cx3cr1_−/− (C) animals exhibited a gene dose-dependent reduction in fibrillar Aβ deposition throughout the cortex and hippocampus. Quantification of Thioflavine S staining in the cortex (D) and hippocampus (E) across all animals revealed a statistically significant decrease in APPPS1 mice with either one or two copies of Cx3cr1 loss of function alleles when compared with age-matched APPPS1;Cx3cr1+/+ controls (*P < 0.001). Notably, there was also a significant difference in Aβ deposition in the cortex between APPPS1;Cx3cr1+/− and APPPS1;_Cx3cr1_−/− genotypes (P < 0.05). To confirm results obtained using Thioflavine S staining, Western blots of brain extracts from Cx3cr1+/+, APPPS1;Cx3cr1+/+, and APPPS1;_Cx3cr1_−/− animals were probed with antibodies to human Aβ and GAPDH as a loading control. Consistent with Thioflavine S staining, APPPS1;_Cx3cr1_−/− brains exhibited lower steady-state Aβ levels when compared with age-matched APPPS1;Cx3cr1+/+ controls (F). Furthermore, ELISAs performed on APPPS1;Cx3cr1+/+ (n = 6) and APPPS1;_Cx3cr1_−/− brain homogenates (n = 5) revealed reduced Aβ42 levels in CX3CR1-deficient animals (G; *P < 0.05).
Figure 2
Lack of effect of CX3CR1 genotype on APP processing. A: Western blots of brain extracts from the APPPS1;Cx3cr1+/+ (Lanes 1–3), APPPS1;Cx3cr1+/− (Lanes 4–6). and APPPS1;_Cx3cr1_−/− (Lanes 7–9) animals were probed with an antibody to the C terminus of APP (top two panels) and subsequently stripped and reprobed with an antibody against α-tubulin as a loading control. Shown on the right is the approximate size in kDa. B: Relative levels of holo-APP were quantified (n = 8) from each genotype by normalizing the intensity values of APP to α-tubulin. C: Relative values of CTFβ were quantified similarly averaging the values of APP CTFβ to α-tubulin. No significant differences were observed in the relative levels of either holo-APP or CTFβ between genotypes.
Figure 3
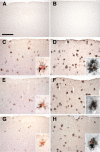
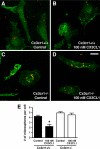
Altered expression of microglial markers in the APPPS1 mouse model of AD with CX3CR1 deficiency. Brain sections (30 μm) from wild-type C57BL/6J (A and B), APPPS1;Cx3cr1+/+ (C and D), APPPS1;Cx3cr1+/− (E and F), and APPPS1;_Cx3cr1_−/− (G and H) mice at 4 months of age were immunostained with antibodies to either CD68, a marker for phagocytic microglia/macrophages (A, C, E, and G), or CD45, a marker for cells of hematopoietic lineage which is up-regulated in activated microglia (B, D, F, and H). Sections were also counterstained with Congo Red, a dye specific for dense-core Aβ deposits. Wild-type sections contained no Congo Red–positive deposits and rare CD68 (A) and CD45 (B) immunoreactivity. Consistent with the published literature, APPPS1;Cx3cr1+/+ animals show abundant CD68 (C) and CD45 immunoreactivity (D), especially in the immediate vicinity of Congo Red–positive Aβ deposits (insets). While age-matched APPPS1;Cx3cr1+/− and APPPS1;_Cx3cr1_−/− animals exhibit gene dose-dependent reduction in CD68 immunoreactivity compared to APPPS1;Cx3cr1+/+ controls (E and G, respectively), CD45 immunoreactivity is relatively unchanged in APPPS1;Cx3cr1+/− and APPPS1;_Cx3cr1_−/− animals compared with APPPS1;Cx3cr1+/+ controls (F and H, respectively). As expected, higher magnification revealed that CD68- and CD45-positive microglia are mostly clustered around Congo Red–positive Aβ deposits (insets). Scale bar = 250 μm.
Figure 4
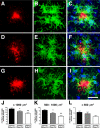
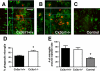
Reduced plaque-associated microglia in the APPPS1 mouse model of AD with CX3CR1 deficiency. Brain sections (30 μm) from APPPS1;Cx3cr1+/+ (A–C), APPPS1;Cx3cr1+/− (D–F), and APPPS1;_Cx3cr1_−/− (G–I) mice at 4 months of age were immunostained with monoclonal Aβ antibody 4G8 (red; A, D, and G), with an antibody against the pan-microglial marker Iba1 (green; B, E, and H), and counterstained with the nuclear TO-PRO-3 dye (blue, C, F, and I). Confocal microscopy was used to obtain maximum projections reconstructed from Z-stacks spanning 20–30 μm in depth. As expected, APPPS1;Cx3cr1+/+ controls exhibit extensive accumulation of Iba1-positive microglia around senile plaques (A–C). However, age-matched APPPS1;Cx3cr1+/− (D–F) and APPPS1;_Cx3cr1_−/− animals (G–I) exhibit gene dose-dependent reduction in the number of Iba1-positive microglia surrounding the senile plaques. The number of Iba1-positive microglia associated with senile plaques in the three genotypes was quantified in three nonadjacent sections from each of the four animals per genotype. APPPS1 mice with either one or two copies of Cx3cr1 loss-of-function alleles exhibited a statistically significant reduction in microglia surrounding both large (>1000 μm2; J), medium (>500 μm2, <1000 μm2; K), and small (<500 μm2; L) Aβ deposits when compared to age-matched APPPS1;Cx3cr1+/+ controls (*P < 0.05; **P < 0.001, respectively). The APPPS1;Cx3cr1+/− and APPPS1;_Cx3cr1_−/− genotypes exhibited a statistically significant difference in microglial accumulation around medium Aβ deposits (P < 0.05), suggesting a gene dose-dependent effect. n = 35 for all analyses. Scale bar = 25 μm.
Figure 5
Altered expression of inflammatory markers in CX3CR1-deficient APPPS1 mouse model of AD. Relative mRNA expression of TNFα (A), IL1β (B), and MCP-1 (C) in APPPS1;Cx3cr1+/+ (n = 7), APPPS1;Cx3cr1+/− (n = 8), and APPPS1;_Cx3cr1_−/− (n = 9) mice at 4 months. The mRNA levels for each cytokine were normalized to the mRNA levels of GAPDH and expressed relative to that of nontransgenic C57BL/6J mice. Results from APPPS1;Cx3cr1+/− and APPPS1;_Cx3cr1_−/− animals were compared with results from age-matched APPPS1;Cx3cr1+/+ controls (*P < 0.05, **P < 0.01, ***P < 0.001).
Figure 6
CX3CL1-CX3CR1 signaling inhibits microglial phagocytosis. Cx3cr1+/+ (A and B) and _Cx3cr1_−/− (C and D) primary microglia were incubated in the absence (A and C) or presence (B and D) of CX3CL1 (100 nmol/L) overnight, and fluorescent microspheres were added for 90 minutes. The cells were fixed and stained with antibody against Iba1 and visualized with Alexa 648-conjugated secondary antibody (pseudo-colored green; A–D). Single-plane confocal images are shown. CX3CL1-treated Cx3cr1+/+ microglia exhibited reduced numbers of phagocytosed microspheres per cell when compared with untreated Cx3cr1+/+ and CX3CL1-treated _Cx3cr1_−/− microglia (E; *P < 0.05). n = 3 for each group. Scale bar = 15 μm.
Figure 7
Enhanced Aβ phagocytosis in CX3CR1-deficient mice. Five- to 7-month-old Cx3cr1+/+ (n = 4; A) and _Cx3cr1_−/− (n = 4; B) mice were injected with fibrillar Aβ1-42 (red; HiLyte 555-conjugated) or with control Aβ42-1 peptide (n = 6; C). Sections (30 μm) containing the needle track were immunostained with an antibody against Iba1 and visualized with Alexa 648-conjugated secondary antibody (pseudo-colored green; A–C). From confocal Z-stacks spanning 20–30 μm in depth, we obtained maximum projections (A–C) and slices in the x- and y-plane showing Aβ internalization (A and B). The number of microglia (E) and percent phagocytic microglia (D) within 50 μm of the needle track were quantified. _Cx3cr1_−/− microglia exhibited enhanced Aβ phagocytosis when compared with control Cx3cr1+/+ microglia (*P < 0.01; D), whereas the number of microglia around the injection site did not significantly differ between genotypes (E). Furthermore, as expected, control Aβ42-1 peptide injection triggered significantly reduced microglial reaction compared to fibrillar Aβ1-42 injection (C and E; *P < 0.05).
Similar articles
- Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis.
Liu Z, Condello C, Schain A, Harb R, Grutzendler J. Liu Z, et al. J Neurosci. 2010 Dec 15;30(50):17091-101. doi: 10.1523/JNEUROSCI.4403-10.2010. J Neurosci. 2010. PMID: 21159979 Free PMC article. - Heterozygous CX3CR1 Deficiency in Microglia Restores Neuronal β-Amyloid Clearance Pathways and Slows Progression of Alzheimer's Like-Disease in PS1-APP Mice.
Hickman SE, Allison EK, Coleman U, Kingery-Gallagher ND, El Khoury J. Hickman SE, et al. Front Immunol. 2019 Dec 2;10:2780. doi: 10.3389/fimmu.2019.02780. eCollection 2019. Front Immunol. 2019. PMID: 31849963 Free PMC article. - Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway.
Lee S, Xu G, Jay TR, Bhatta S, Kim KW, Jung S, Landreth GE, Ransohoff RM, Lamb BT. Lee S, et al. J Neurosci. 2014 Sep 10;34(37):12538-46. doi: 10.1523/JNEUROSCI.0853-14.2014. J Neurosci. 2014. PMID: 25209291 Free PMC article. - CX3CL1 Pathway as a Molecular Target for Treatment Strategies in Alzheimer's Disease.
Bivona G, Iemmolo M, Ghersi G. Bivona G, et al. Int J Mol Sci. 2023 May 4;24(9):8230. doi: 10.3390/ijms24098230. Int J Mol Sci. 2023. PMID: 37175935 Free PMC article. Review.
Cited by
- Microglia Susceptibility to Free Bilirubin Is Age-Dependent.
Vaz AR, Falcão AS, Scarpa E, Semproni C, Brites D. Vaz AR, et al. Front Pharmacol. 2020 Jul 14;11:1012. doi: 10.3389/fphar.2020.01012. eCollection 2020. Front Pharmacol. 2020. PMID: 32765258 Free PMC article. - G Protein-Coupled Receptors (GPCRs) in Alzheimer's Disease: A Focus on BACE1 Related GPCRs.
Zhao J, Deng Y, Jiang Z, Qing H. Zhao J, et al. Front Aging Neurosci. 2016 Mar 24;8:58. doi: 10.3389/fnagi.2016.00058. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27047374 Free PMC article. Review. - Trem2 deletion enhances tau dispersion and pathology through microglia exosomes.
Zhu B, Liu Y, Hwang S, Archuleta K, Huang H, Campos A, Murad R, Piña-Crespo J, Xu H, Huang TY. Zhu B, et al. Mol Neurodegener. 2022 Sep 2;17(1):58. doi: 10.1186/s13024-022-00562-8. Mol Neurodegener. 2022. PMID: 36056435 Free PMC article. - Neuroinflammation in Prion Disease.
Li B, Chen M, Zhu C. Li B, et al. Int J Mol Sci. 2021 Feb 23;22(4):2196. doi: 10.3390/ijms22042196. Int J Mol Sci. 2021. PMID: 33672129 Free PMC article. Review. - Peli1 impairs microglial Aβ phagocytosis through promoting C/EBPβ degradation.
Xu J, Yu T, Pietronigro EC, Yuan J, Arioli J, Pei Y, Luo X, Ye J, Constantin G, Mao C, Xiao Y. Xu J, et al. PLoS Biol. 2020 Oct 5;18(10):e3000837. doi: 10.1371/journal.pbio.3000837. eCollection 2020 Oct. PLoS Biol. 2020. PMID: 33017390 Free PMC article.
References
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease–a double-edged sword. Neuron. 2002;35:419–432. - PubMed
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG023012/AG/NIA NIH HHS/United States
- F30NS068003/NS/NINDS NIH HHS/United States
- F30 NS068003/NS/NINDS NIH HHS/United States
- R01 AG023012/AG/NIA NIH HHS/United States
- T32 GM007250/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous