Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway - PubMed (original) (raw)
Methylated H3K4, a transcription-associated histone modification, is involved in the DNA damage response pathway
David Faucher et al. PLoS Genet. 2010.
Abstract
Eukaryotic genomes are associated with a number of proteins such as histones that constitute chromatin. Post-translational histone modifications are associated with regulatory aspects executed by chromatin and all transactions on genomic DNA are dependent on them. Thus, it will be relevant to understand how histone modifications affect genome functions. Here we show that the mono ubiquitylation of histone H2B and the tri-methylation of histone H3 on lysine 4 (H3K4me3), both known for their involvement in transcription, are also important for a proper response of budding yeast cells to DNA damaging agents and the passage through S-phase. Cells that cannot methylate H3K4 display a defect in double-strand break (DSB) repair by non-homologous end joining. Furthermore, if such cells incur DNA damage or encounter a stress during replication, they very rapidly lose viability, underscoring the functional importance of the modification. Remarkably, the Set1p methyltransferase as well as the H3K4me3 mark become detectable on a newly created DSB. This recruitment of Set1p to the DSB is dependent on the presence of the RSC complex, arguing for a contribution in the ensuing DNA damage repair process. Taken together, our results demonstrate that Set1p and its substrate H3K4me3, which has been reported to be important for the transcription of active genes, also plays an important role in genome stability of yeast cells. Given the high degree of conservation for the methyltransferase and the histone mark in a broad variety of organisms, these results could have similar implications for genome stability mechanisms in vertebrate and mammalian cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
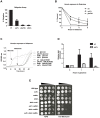
Figure 1. Genetic interactions between a histone modification pathway and the MRX complex.
(A) Tetrad analysis of spores derived from diploids micro-dissected on YEPD plates and allowed to grow for 3 days at 23°C. Circled colonies are double mutants. Diploids DFY001, DFY002, DFY004 and DFY005 respectively, were used as starting strains. (B–F) Serial ten-fold dilution growth tests of exponentially growing cultures on plates with the indicated compounds. YEPD: control plates without drug; (B) Growth of cells with a deletion of members of the Ubiquitin-Ligase complex in combination with a deletion of RAD50 (spores derived from diploids DFY002 and DFY007); (C) Spot dilution test of _bre1_Δ _tel1_Δ double mutant cells on plates containing the indicated concentration of Bleomycin (right) or no drug (left, spores were derived from DFY005); (D) Growth of cells with a mutant that cannot be mono-ubiquitylated (H2BK123R) (strains YZS276, YZS246, DFY008, DFY009 and MT0-73); (E) Growth of cells with deletions of DOT1 or SET1 genes in combination with absence of RAD50 (spores derived from diploids DFY010 and DFY012); and (F) Growth of cells with a mutant histone H3 that cannot be methylated (H3K79R or H3K4R) and combined with an absence of RAD50 (strains YZS267, DFY013, DFY015, DFY016, DFY017 and DFY018).
Figure 2. The LGE1-BRE1-SET1 histone modification pathway is involved in DSB repair by non-homologous end joining (NHEJ).
(A) _Set1_Δ cells display a deficiency in a plasmid religation assay. Linearized plasmid pRS316 was transformed into wild type cells (wt, YW1276), _set1_Δ (DFY021), _yku70_Δ (YW1283) or _dnl4_Δ cells (DFY022). Overall percentage of religation was calculated using transformation efficiency normalization. (B) Survival of wt (JKM179), _set1_Δ (DFY023) or _yku70_Δ cells (JKM181) following induction of a DSB. Cells were held in galactose media for the indicated time and then plated on glucose medium, shutting off HO endonuclease expression. In these strains, the induced DSB at the mating type locus can only be repaired by NHEJ. (C) Growth curves of wt (YW1276), _set1_Δ (DFY021), _yku70_Δ (YW1283), and _dnl4_Δ cells (DFY022) exposed to a constant expression of HO endonuclease. (D) Recruitment of Yku80-myc protein to an induced DSB site in wt (DFY048) and _set1_Δ (DFY049) cells. DSB induction was performed for 1 and 3 hours, ChIP performed with an anti-myc antibody and immunoprecipitated DNA quantified by Q-PCR. Fold enrichment is normalized to non-induced cells (glucose) set as 1. (E) Sensitivity to DNA damage of cells harbouring deletions of genes affecting, respectively, NHEJ (DNL4) or homologous recombination (RAD52) when combined with a deletion of SET1. Strains used were spores of DFY020.
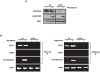
Figure 3. Site-specific recruitment of the Set1p methyltransferase and detection of H3K4me3 at a DSB.
(A) Western blot of proteins derived from wild type and _set1_Δ cells that were treated (+) or not (−) with phleomycin. Top: Tri-methylated H3K4 was detected with an antibody against H3K4me3; middle: detection of phosphorylated H2AS129 as a positive control for phleomycin treatment; and bottom: anti-Pgk1p antibody as a loading control. Strains used were wt DFY024 and _set1_ΔDFY026). (B) Left: Recruitment of HA-tagged Set1p methyltransferase to the mating type locus was tested by ChIP analysis using an anti-HA antibody. HO endonuclease was expressed for 90 min in G1-arrested wt (DFY027) or _bre1_Δ cells (DFY028). DNA was extracted from immunoprecipitates and PCR amplified with specific primers adjacent to the HO cleavage site, primers in the promoter region of the highly expressed gene PYK1, or primers specific for the transcriptionally repressed CEN4 locus. Right: Same experiment as in left panel, except that instead of the anti-HA antibody, an antibody specific for H3K4me3 was used for the immunoprecipitation.
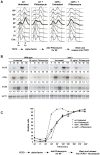
Figure 4. _Set1_Δ cells exposed to DNA damage in G1 are retained in S-phase.
(A) wt (DFY024) and _set1_Δ cells (DFY026) were arrested in G1 phase of the cell cycle and exposed to phleomycin (see experimental scheme at bottom). Cells were then released and cell cycle progression was monitored by flow cytometry of samples at regular intervals. Time 0 min represents the release of cells into rich medium immediately following the phleomycin treatment. (B) In a separate release experiment, northern blot analysis was performed in order to confirm the expression of cell-cycle specific mRNAs: Cln2 for G1, Hta1 for S-phase and Clb2 for G2/M specific RNA expression. A probe specific for the Act1-mRNA was used as a non-varying control (bottom). The number below each lane indicates the change of the signal intensity for the particular RNA; the level at time 0 was set as 1. (C) Budding indexes of wt (DFY024) and _set1_Δ cells (DFY026) subjected to a cell synchrony and release experiment as outlined below the curves. Note that the final media did contain Nocodazole to prevent cells to traverse multiple cycles and that explains why the indexes stay high at the end of the experiment.
Figure 5. Replication defects in _set1_Δ cells.
(A) Serial ten-fold dilution growth test of strains with the relevant genetic setup on plates with 50mM HU. Strains used were YZS267, DFY017, DFY038, DFY039, DFY024, DFY026 and MT0-73. (B) Viability curves of the indicated strains exposed to HU for the indicated time. Strains used were wt (DFY024), _set1_Δ (DFY026), _arp8_Δ (DFY030), _mec1_Δ (DFY044). (C) wt (DFY024), _set1_Δ (DFY026)or _rsc30_Δ (DFY029) cells were arrested in G1 phase of the cell cycle and released into a synchronous S-phase in the presence of 200 mM HU for 60 min. The HU block was removed by washout and cell cycle progression was analyzed by FACS. Time 0 min represents the release of cells into rich medium plus Nocodazole following the HU treatment. (D) Viability of strains with indicated genotype and derived from strain DFY032 were grown in liquid media and ten-fold serial dilutions were plated onto YEPD control plates or onto a plate containing bleomycin, as indicated.
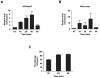
Figure 6. Functional interactions between the RSC complex and SET1.
(A) Example of a spot dilution - colony viability assay performed to unc subunits RSC30 in (B) and RSC1 in (C), in combination with a deletion of RAD50 and/or SET1 (spores derived from diploid DFY034 and DFY050 respectively). Serial ten-fold dilution growth tests of exponentially growing cultures on plates containing DNA damaging agent bleomycin. YEPD: control plates without treatment. (D) ChIP experiments assessing the presence of Set1p in wt cells (DFY027) or in cells lacking either BRE1 (DFY028) or RSC30 (DFY037). (E) Western blot analysis detecting the presence of H3K4me3 in strains harbouring deletions of RSC complex subunit genes. A _set1_Δ–strain serves as negative control and re-hybridization with and anti-Pgk1p antibody was used as a loading control. Strains used were spores of diploid DFY034 and DFY050. (F) ChIP of an essential RSC-component, Sth1p, in cells lacking SET1. Strain used was DFY046. ChIP experiments essentially were conducted as described in Figure 3 over genes displaying a similar DNA damage sensitivity enhancement as _set1_Δ in absence of homologous recombination. Note that _yng1_Δ scored here is negative, the _bre1Δ rad50_Δ serves as positive control; strains used are spores of DFY033. (B,C) Growth of cells with a deletion of RSC complex.
Figure 7. Kinetics of association of H3K4me3 and Sth1p of the RSC complex with an induced DSB.
(A,B) ChIP analysis showing the recruitment of H3K4me3 and Sth1p respectively to an induced DSB. Wild-type cells (DFY046) were exposed to galactose for the indicated period in order to induce HO endonuclease. DNA was extracted from immunoprecipitates and analyzed by quantitative PCR using specific primers adjacent to the HO cleavage site. (C) Analysis of the HO cleavage efficiency of cells used for ChIP analysis in (A,B) using Q-PCR. DNA was extracted from cells and Q-PCR with primers overlapping the DSB was performed. Time 0 (glucose) was set to 0% cleavage efficiency.
Similar articles
- Histone 3 lysine 4 monomethylation supports activation of transcription in S. cerevisiae during nutrient stress.
Deshpande N, Jordan R, Henderson Pozzi M, Bryk M. Deshpande N, et al. Curr Genet. 2022 Apr;68(2):181-194. doi: 10.1007/s00294-022-01226-2. Epub 2022 Jan 18. Curr Genet. 2022. PMID: 35041077 Free PMC article. - H3 lysine 4 is acetylated at active gene promoters and is regulated by H3 lysine 4 methylation.
Guillemette B, Drogaris P, Lin HH, Armstrong H, Hiragami-Hamada K, Imhof A, Bonneil E, Thibault P, Verreault A, Festenstein RJ. Guillemette B, et al. PLoS Genet. 2011 Mar;7(3):e1001354. doi: 10.1371/journal.pgen.1001354. Epub 2011 Mar 31. PLoS Genet. 2011. PMID: 21483810 Free PMC article. - Two distinct repressive mechanisms for histone 3 lysine 4 methylation through promoting 3'-end antisense transcription.
Margaritis T, Oreal V, Brabers N, Maestroni L, Vitaliano-Prunier A, Benschop JJ, van Hooff S, van Leenen D, Dargemont C, Géli V, Holstege FC. Margaritis T, et al. PLoS Genet. 2012 Sep;8(9):e1002952. doi: 10.1371/journal.pgen.1002952. Epub 2012 Sep 20. PLoS Genet. 2012. PMID: 23028359 Free PMC article. - Ready, SET, Go: Post-translational regulation of the histone lysine methylation network in budding yeast.
Separovich RJ, Wilkins MR. Separovich RJ, et al. J Biol Chem. 2021 Aug;297(2):100939. doi: 10.1016/j.jbc.2021.100939. Epub 2021 Jul 3. J Biol Chem. 2021. PMID: 34224729 Free PMC article. Review. - Diverse and dynamic forms of gene regulation by the S. cerevisiae histone methyltransferase Set1.
Deshpande N, Bryk M. Deshpande N, et al. Curr Genet. 2023 Jun;69(2-3):91-114. doi: 10.1007/s00294-023-01265-3. Epub 2023 Mar 31. Curr Genet. 2023. PMID: 37000206 Review.
Cited by
- H3K4me2 regulates the recovery of protein biosynthesis and homeostasis following DNA damage.
Wang S, Meyer DH, Schumacher B. Wang S, et al. Nat Struct Mol Biol. 2020 Dec;27(12):1165-1177. doi: 10.1038/s41594-020-00513-1. Epub 2020 Oct 12. Nat Struct Mol Biol. 2020. PMID: 33046905 - Characterization of histone modifications associated with DNA damage repair genes upon exposure to gamma rays in Arabidopsis seedlings.
Mondal S, Go YS, Lee SS, Chung BY, Kim JH. Mondal S, et al. J Radiat Res. 2016 Nov;57(6):646-654. doi: 10.1093/jrr/rrw077. Epub 2016 Aug 16. J Radiat Res. 2016. PMID: 27534791 Free PMC article. - Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression.
Baradaran-Heravi A, Cho KS, Tolhuis B, Sanyal M, Morozova O, Morimoto M, Elizondo LI, Bridgewater D, Lubieniecka J, Beirnes K, Myung C, Leung D, Fam HK, Choi K, Huang Y, Dionis KY, Zonana J, Keller K, Stenzel P, Mayfield C, Lücke T, Bokenkamp A, Marra MA, van Lohuizen M, Lewis DB, Shaw C, Boerkoel CF. Baradaran-Heravi A, et al. Hum Mol Genet. 2012 Jun 1;21(11):2572-87. doi: 10.1093/hmg/dds083. Epub 2012 Feb 28. Hum Mol Genet. 2012. PMID: 22378147 Free PMC article. - Chromatin modifications and the DNA damage response to ionizing radiation.
Kumar R, Horikoshi N, Singh M, Gupta A, Misra HS, Albuquerque K, Hunt CR, Pandita TK. Kumar R, et al. Front Oncol. 2013 Jan 22;2:214. doi: 10.3389/fonc.2012.00214. eCollection 2012. Front Oncol. 2013. PMID: 23346550 Free PMC article. - The COMPASS subunit Spp1 protects nascent DNA at the Tus/Ter replication fork barrier by limiting DNA availability to nucleases.
Ghaddar N, Corda Y, Luciano P, Galli M, Doksani Y, Géli V. Ghaddar N, et al. Nat Commun. 2023 Sep 5;14(1):5430. doi: 10.1038/s41467-023-41100-4. Nat Commun. 2023. PMID: 37669924 Free PMC article.
References
- Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. - PubMed
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. - PubMed
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. - PubMed
- Sanchez Y, Zhou Z, Huang M, Kemp BE, Elledge SJ. Analysis of budding yeast kinases controlled by DNA damage. Methods in Enzymology. 1997;283:398–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases