Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1 - PubMed (original) (raw)
Autoamplification of Notch signaling in macrophages by TLR-induced and RBP-J-dependent induction of Jagged1
Julia Foldi et al. J Immunol. 2010.
Abstract
Several signaling pathways, including the Notch pathway, can modulate TLR activation to achieve responses most appropriate for the environment. One mechanism of TLR-Notch cross-talk is TLR-induced expression of Notch ligands Jagged and Delta that feed back to engage Notch receptors on TLR-activated cells. In this study, we investigated mechanisms by which TLRs induce Notch ligand expression in primary macrophages. TLRs induced Jagged1 expression rapidly and independently of new protein synthesis. Jagged1 induction was augmented by IFN-γ, was partially dependent on canonical TLR-activated NF-κB and MAPK signaling pathways, and elevated Jagged1 expression augmented TLR-induced IL-6 production. Strikingly, TLR-induced Jagged1 expression was strongly dependent on the Notch master transcriptional regulator RBP-J and also on upstream components of the Notch pathway γ-secretase and Notch1 and Notch2 receptors. Thus, Jagged1 is an RBP-J target gene that is activated in a binary manner by TLR and Notch pathways. Early and direct cooperation between TLR and Notch pathways leads to Jagged1-RBP-J-mediated autoamplification of Notch signaling that can modulate later phases of the TLR response.
Figures
Figure 1
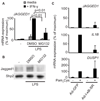
Jagged1 is a direct TLR target gene and is superinduced by IFN-γ (A) Primary human macrophages were stimulated with TLR ligands LPS (1 ng/ml) or Pam3Cys (10 ng/ml) for 3 h. mRNA expression was measured by qRT-PCR and normalized relative to the level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. Relative mRNA levels are expressed as percentage of GAPDH mRNA for each human donor and means are indicated for each condition. Data shown are pooled from 63 (untreated), 39 (LPS treatment) or 29 (Pam3Cys treatment) independent human donors. Statistical analysis was performed using the unpaired Student’s t test. (B) Human primary macrophages isolated from four donors were stimulated with LPS for the indicated times and mRNA was measured. Data are graphed as the percentage of maximum mRNA expression after 1 h LPS stimulation. Statistical analysis was performed using the paired Student’s t test. (C) Jagged1 mRNA measured from human macrophages treated with the protein synthesis inhibitor, cycloheximide (CHX, 20 µg/ml) for 30 min before stimulation with LPS for 1 h. Data are expressed as means + SD of three independent experiments and statistical analysis was performed using the paired Student’s t test. (D) Flow cytometric analysis of Jagged1 surface expression from human macrophages treated with LPS for the indicated times. Data shown are from one representative donor out of more than five. Isotype control staining is shown by the shaded plot, Jagged1 staining by the open line. Corrected mean fluorescence intensities (Jagged1-isotype) are indicated. (E) Jagged1 mRNA from human macrophages primed with 100 U/ml IFN-γ and stimulated with LPS for 3 h. (F and G) Human macrophages were primed with IFN-γ prior to stimulation with LPS and mRNA (F) and protein (G) were measured. (H) Human macrophages isolated from six donors were primed with IFN-γ prior to stimulation with LPS for 3 h. Primary transcript levels were measured using qRT-PCR and primers designed for sequences located in the intronic regions of the JAGGED1 gene. Data are expressed as percentage of maximum mRNA expression, which corresponds to the IFN-γ- and LPS-treated condition. Statistical analysis was performed using the paired Student’s t test.
Figure 2
TLR induction of Jagged1 is dependent on the NF-κB pathway in human macrophages. (A) Human primary macrophages isolated from four donors were primed with IFN-γ for 20 h. A proteasome inhibitor (MG-132, 20 µM) or vehicle control, dimethyl sulfoxide (DMSO) were added to the cells one hour prior to stimulation with LPS for 3 h and mRNA was measured. Data are expressed as means + SD of four independent experiments and statistical analysis was performed using the paired Student’s t test. (B) Human macrophages were treated with MG-132 for 1 h prior to stimulation with LPS for 6 h. Total protein lysates were blotted with antibodies specific for Jagged1 and Shp2. (C) Human macrophages isolated from two donors were infected with an adenovirus expressing a phosphorylation-resistant super repressor of I-kBα (Ad-I-κB-SR) or control adenovirus encoding GFP (Ad-GFP). Cells were then stimulated with Pam3Cys for 3 h and mRNA was measured.
Figure 3
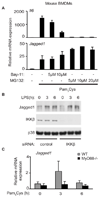
The role of canonical TLR signaling components in Jagged1 induction in murine macrophages. (A) Mouse BMDMs from WT C57B/L6 mice were pretreated with the IKK-inhibitor, Bay-11 (20 µM) or the proteasome inhibitor, MG-132, at different concentrations, for 30 min. Cells were then stimulated with Pam3Cys for 3 h and mRNA was measured. Data shown are representative of three independent experiments. (B) Mouse BMDMs were transfected with siRNA against IKKβ or control, non-targeting siRNA. 72 hours post-transfection, cells were stimulated with LPS and whole cell lysates were immunoblotted with antibodies against Jagged1, IKKβ and p38. (C) BMDMs from two WT and two MyD88−/− mice were stimulated with Pam3Cys for 3 h and mRNA expression of Jagged1 was measured.
Figure 4
Jagged1 expression is dependent on the Notch pathway component RBP-J. (A) Mouse BMDMs from RBP-Jfl/fl, Cre and RBP-J+/+, Cre littermate controls were stimulated with LPS for the indicated times and mRNA was measured. (B) BMDMs were primed with 100 U/ml IFN-γ for 20 h prior to stimulation with LPS for 6 h. Total protein was imunoblotted with antibodies against Jagged1 and Shp2. Data shown are representative of five experiments. (C) Human macrophages were transfected with siRNA against RBP-J or control, non-targeting siRNA. 72 hours post-transfection, cells were stimulated with TLR-L (LPS or Pam3Cys) for 3 h and mRNA was measured. Data are shown as percent of maximal mRNA expression of the TLR-L stimulated control cells, and are expressed as means + SD of four independent experiments. Statistical analysis was performed using the paired Student’s t test.
Figure 5
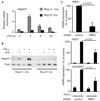
Notch signaling contributes to TLR-induced Jagged1 expression. (A and B) Human primary macrophages were treated with either vehicle control or the γ-secretase inhibitor, GSI-34 (10µM) for 48 h prior to stimulation with Pam3Cys for (A) 3 h or for (B) 6 h. Jagged1 was measured by (A) qRT-PCR or by (B) immunoblot. Statistical analysis was performed using the paired Student’s t test. (C) Human macrophages were transfected with siRNA’s against both Notch1 and Notch2 (Notch1/2) or control, non-targeting siRNA. 72 hours post-transfection, cells were primed with IFNγ and stimulated with LPS for 3 h and mRNA was measured. Data are shown as percent of maximal mRNA expression of control cells, and are expressed as means + SD of three independent experiments. Statistical analysis was performed using the paired Student’s t test. (D) The human WT Jagged1 promoter, a truncated (Trunc) version of the promoter, which lacks the conserved NF-κB site at −3034 and a mutant promoter in which the NF-κB site was mutated (NF-κB mut), were transfected into RAW264.7 cells and co-transfected in triplicate with increasing amounts of an NICD1 expression plasmid or empty vector. 24 h after transfection, cell lysates were analyzed for luciferase activity. Data shown are representative of three independent experiments. There was no statistically significant difference between the induction of the three promoter constructs by NICD1.
Figure 6
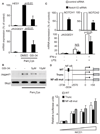
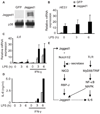
Jagged1 contributes to TLR-induced IL-6 expression. THP1 cells were stably transduced with a lentivirus expressing full length human Jagged1 or a control lentivirus expressing GFP. (A) Immunoblot of whole cell lysates from GFP- or Jagged1-expressing THP1 cells. (B and C) THP1 cells were primed with 100 U/ml IFN-γ for 20 h followed by stimulation with 1 µg/ml LPS for the indicated times. (D) ELISA of culture supernatants from THP1 cells differentiated into macrophages using 0.5 µM PMA overnight followed by stimulation as in (B and C). Data shown are representative of four experiments. (E) In primary human macrophages, basal signaling through the constitutively expressed receptors, Notch1 and 2 as well as concomitant activation of NF-κB and MAPK by TLRs are involved in robust induction of Jagged1. Downstream of Notch, the canonical Notch signaling machinery, including cleavage of NICD by γ-secretase and activation of RBPJ, are important, while the signal from TLRs can be mediated by either MyD88 or TRIF, for maximal Jagged1 induction. Notch- and TLR-mediated induction of Jagged1 leads to the amplification of Notch signaling, which also contributes to the production of the cytokine, IL-6.
Similar articles
- Jagged1-selective notch signaling induces smooth muscle differentiation via a RBP-Jkappa-dependent pathway.
Doi H, Iso T, Sato H, Yamazaki M, Matsui H, Tanaka T, Manabe I, Arai M, Nagai R, Kurabayashi M. Doi H, et al. J Biol Chem. 2006 Sep 29;281(39):28555-64. doi: 10.1074/jbc.M602749200. Epub 2006 Jul 25. J Biol Chem. 2006. PMID: 16867989 - Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways.
Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, Tateya T, Kang YJ, Han J, Gessler M, Kageyama R, Ivashkiv LB. Hu X, et al. Immunity. 2008 Nov 14;29(5):691-703. doi: 10.1016/j.immuni.2008.08.016. Epub 2008 Oct 30. Immunity. 2008. PMID: 18976936 Free PMC article. - Deletion of RBP-J in dendritic cells compromises TLR-mediated DC activation accompanied by abnormal cytoskeleton reorganization.
Chen YR, Feng F, Wang L, Qu SY, Zhang ZQ, Liu L, Qin HY, Liang YM, Han H. Chen YR, et al. Mol Biol Rep. 2013 Feb;40(2):1531-9. doi: 10.1007/s11033-012-2198-3. Epub 2012 Nov 9. Mol Biol Rep. 2013. PMID: 23138187 - Nitric oxide is involved in Mycobacterium bovis bacillus Calmette-Guérin-activated Jagged1 and Notch1 signaling.
Kapoor N, Narayana Y, Patil SA, Balaji KN. Kapoor N, et al. J Immunol. 2010 Mar 15;184(6):3117-26. doi: 10.4049/jimmunol.0903174. Epub 2010 Feb 10. J Immunol. 2010. PMID: 20147635 - Notch signalling beyond liver development: emerging concepts in liver repair and oncogenesis.
Morell CM, Fiorotto R, Fabris L, Strazzabosco M. Morell CM, et al. Clin Res Hepatol Gastroenterol. 2013 Nov;37(5):447-54. doi: 10.1016/j.clinre.2013.05.008. Epub 2013 Jun 24. Clin Res Hepatol Gastroenterol. 2013. PMID: 23806629 Review.
Cited by
- Notch Signaling in Breast Tumor Microenvironment as Mediator of Drug Resistance.
Chimento A, D'Amico M, Pezzi V, De Amicis F. Chimento A, et al. Int J Mol Sci. 2022 Jun 4;23(11):6296. doi: 10.3390/ijms23116296. Int J Mol Sci. 2022. PMID: 35682974 Free PMC article. Review. - Myeloid-Specific Blockade of Notch Signaling by RBP-J Knockout Attenuates Spinal Cord Injury Accompanied by Compromised Inflammation Response in Mice.
Chen BY, Zheng MH, Chen Y, Du YL, Sun XL, Zhang X, Duan L, Gao F, Liang L, Qin HY, Luo ZJ, Han H. Chen BY, et al. Mol Neurobiol. 2015 Dec;52(3):1378-1390. doi: 10.1007/s12035-014-8934-z. Epub 2014 Oct 26. Mol Neurobiol. 2015. PMID: 25344316 - Macrophages in Calcific Aortic Valve Disease: Paracrine and Juxtacrine Disease Drivers.
Klauzen P, Basovich L, Shishkova D, Markova V, Malashicheva A. Klauzen P, et al. Biomolecules. 2024 Dec 2;14(12):1547. doi: 10.3390/biom14121547. Biomolecules. 2024. PMID: 39766254 Free PMC article. Review. - Notch signaling in developmental and tumor angiogenesis.
Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Kofler NM, et al. Genes Cancer. 2011 Dec;2(12):1106-16. doi: 10.1177/1947601911423030. Genes Cancer. 2011. PMID: 22866202 Free PMC article. - The role of the Notch signalling pathway in the pathogenesis of ulcerative colitis: from the perspective of intestinal mucosal barrier.
Ning H, Liu J, Tan J, Yi M, Lin X. Ning H, et al. Front Med (Lausanne). 2024 Jan 5;10:1333531. doi: 10.3389/fmed.2023.1333531. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38249980 Free PMC article. Review.
References
- Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. - PubMed
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
- Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. - PubMed
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. - PubMed
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI044938/AI/NIAID NIH HHS/United States
- R01 AR050401/AR/NIAMS NIH HHS/United States
- R01 AR056729/AR/NIAMS NIH HHS/United States
- R01 HL062454/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous