Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis - PubMed (original) (raw)
Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis
Pengcheng Wang et al. Plant Cell. 2010 Sep.
Abstract
Nitric oxide (NO) is a bioactive molecule that functions in numerous physiological and developmental processes in plants, including lateral root development. In this study, we used biochemical and genetic approaches to analyze the function of Arabidopsis thaliana mitogen-activated protein kinase 6 (MPK6) in the regulation of NO synthesis in response to hydrogen peroxide (H₂O₂) during lateral root development. In both mpk6 mutants studied, H₂O₂-induced NO synthesis and nitrate reductase (NR) activity were decreased dramatically. Furthermore, one NR isoform, NIA2, was required for the MPK6-mediated production of NO induced by H₂O₂. Notably, NIA2 interacted physically with MPK6 in vitro and in vivo and also served as a substrate of MPK6. Phosphorylation of NIA2 by MPK6 led to an increase in NR activity, and Ser-627 was identified as the putative phosphorylation site on NIA2. Phenotypical analysis revealed that mpk6-2 and mpk6-3 seedlings produce more and longer lateral roots than wild-type plants did after application of the NO donor sodium nitroprusside or H₂O₂. These data support strongly a function of MPK6 in modulating NO production and signal transduction in response to H₂O₂ during Arabidopsis root development.
Figures
Figure 1.
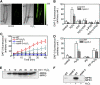
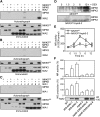
mpk6 Mutants Produce Less Nitric Oxide than Wild-Type Plants in Response to H2O2. (A) Images of H2O2-induced NO production in wild-type and mpk6-3 plants. Roots from wild-type and mpk6-3 mutants were loaded with DAF-2 DA, and NO synthesis was monitored after the addition of 10 μM H2O2 or a water control. The micrographs show pairs of representative bright-field and fluorescence images of the roots of wild-type and mpk6-3 plants in six independent experiments. Bar = 100 μm. (B) Effects of CAT and c-PTIO on DAF-2 fluorescence in wild-type and mpk6-3 plants in the presence and absence of H2O2. Control represents wild-type and mutants treated with water. Average pixel intensities of DAF-2 DA fluorescence were calculated after addition of CAT or c-PTIO for 30 min. Values are means (±
sd
) from whole root regions (from maturation zone to meristematic zone) from six independent experiments. (C) Time course of NO production expressed as the pixel intensities of DAF-2 DA fluorescence in the roots of wild-type and mpk6-3 plants before and after addition of H2O2. Wild-type and mpk6-3 plants were treated with 10 μM H2O2 for the times indicated. Signal intensities were quantified over whole roots from micrographs taken under confocal laser scanning microscopy. Values are means (±
sd
) from six independent experiments. (D) Effects of PD 098059 on NO production in wild-type and mpk6-3 plants in the presence and absence of H2O2. Average pixel intensities of DAF-2 DA fluorescence were calculated after addition of PD 098059, H2O2, or water control for 30 min. Values are means (±
sd
) from whole root regions from six independent experiments. (E) H2O2-induced activation of MPK6 and MPK3 in vivo. Proteins were extracted from wild-type seedlings treated with 2 mM H2O2 for the time indicated. In-gel kinase assays were performed as described in Methods, and MBP was used as the substrate. (F) Mutation of mpk6 abolishes H2O2-activated MPK6. The wild-type and mpk6-3 plants were treated with 2 mM H2O2 for 20 min, and the activity of MPK6 was detected by in-gel kinase assay using MBP as the substrate.
Figure 2.
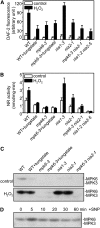
Involvement of NIA2 in MPK6-Modulated NO Biosynthesis in Response to H2O2. (A) NO production in the wild type, mpk6-3, and nia single or mpk6-3 nia2-1 and nia1-2 nia2-5 double mutants. Average pixel intensities of DAF-2 DA fluorescence were measured at 30 min after exposure to exogenous 10 μM H2O2 or a water control. For tungstate treatment, seedlings were loaded with 200 μM tungstate for 30 min before addition of H2O2. Values are means (±
sd
) from whole root regions from six independent experiments. (B) H2O2-induced increase in NR activity in the wild type, mpk6-3, and nia single or mpk6-3 nia2-1 and nia1-2 nia2-5 double mutants. Arabidopsis plants were treated with 10 μM H2O2 or a water control for 30 min. Each value represents the mean (±
sd
) of three independent experiments. (C) Effects of H2O2 on MPK6 phosphorylation in wild-type, mpk6-3, nia, and mpk6-3 nia2-1 mutants. The wild-type and mutant plants were treated with 2 mM H2O2 or a water control for 20 min. For tungstate treatment, seedlings were loaded with 200 μM tungstate for 30 min before addition of H2O2. The activity of MPK6 was detected by in-gel kinase assay using MBP as the substrate. (D) Effects of the NO donor SNP on MPK6 phosphorylation. Proteins were extracted from wild-type seedlings treated with 20 μM SNP at the indicated times. In-gel kinase assays were performed with MBP as the substrate.
Figure 3.
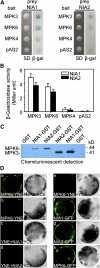
MPK6 Interacts with NIA2 in Vitro and in Vivo. (A) MPK6 and MPK3 interacted with NIA1 and NIA2 in the yeast two-hybrid system. Yeast strains that contained pAS-MPK6 or pAS-MPK3 as bait and pACT-NIA1/2 as prey were grown for 48 h on synthetic defined (SD) medium that lacked Trp and Leu (left panel) and were assayed for LacZ expression by a filter-lift assay for β-galactosidase activity (β-gal; right panel). pAS-MPK4 and the empty bait vector were used as negative controls. A blue color indicates interaction. β-gal, β-galactosidase activity. (B) Quantitative analysis of β-galactosidase activity of the yeast strains in liquid culture showing the interaction between MPK3, MPK6, and MPK4 and NIA1 or NIA2. Values are means of data from three independent experiments. Error bars indicate
sd
. (C) MPK3 and MPK6 proteins labeled with biotinylated-Lys were pulled down by GST-NIA1 and GST-NIA2 but not by GST. (D) NIA2, but not NIA1, interacted with MPK6 in vivo as determined by BiFC. (a) The YFP signal in the cytoplasm indicates a positive interaction between MPK6 and NIA2; (b) no YFP signal was detected in protoplasts cotransformed with MPK6-YNE and NIA1-YCE; (c) and (d) no YFP signal was detected in protoplasts cotransformed with SPYNE and NIA2-YCE or NIA1-YCE; (e) no YFP signal was detected in protoplasts cotransformed with MPK6-YNE and SPYCE; (f) to (h) localization of GFP signals from NIA1, NIA2, and MPK6 fused with GFP. Left panels, fluorescence images under confocal microscopy; right panels, bright-field images of the cells. Bars = 5 μm.
Figure 4.
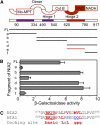
MPK6 Interacts with the Hinge 2 and FAD Domains of NIA2. (A) Schematic representation of seven fragments that covered various regions of the NIA2 protein and were used for yeast two-hybrid assays. FL, full-length NIA2 protein (amino acids 1 to 917). (a) N-terminal region and Mo-molybdopterin domain (amino acids 1 to 334); (b) all regions except those contained in fragment a (amino acids 335 to 917); (c) dimer, hinge 1, and cytochrome b domains (amino acids 335 to 620); (d) hinge 2, FAD, and NADH binding domains (amino acids 621 to 917); (e) hinge 2 and FAD domains (amino acids 621 to 780); (f) NADH binding domain (amino acids 781 to 917). (B) Quantitative analysis of β-galactosidase activity of the yeast strains in liquid culture showing the interaction between MPK6 and the seven fragments of NIA2. Values are means (±
sd
) of data from three independent experiments. The results show that fragment e is required for interaction between MPK6 and NIA2. (C) Sequence of the putative docking domain in NIA1 and NIA2. The first amino acid in each sequence used for alignment is indicated, and conserved residues are highlighted (red). A triplet of conserved hydrophobic residues (MVL) in NIA2 corresponded to QQL in NIA1 (blue). f represents a hydrophobic amino acid.
Figure 5.
Phosphorylation of NIA1 and NIA2 by MPK3 and MPK6. (A) and (B) Activated MPK6 phosphorylated NIA2 in the C-terminal region, but activated MPK3 could not phosphorylate NIA2. Fragments c and d of NIA2 are described in Figure 4 and correspond to amino acids 335 to 620 and 621 to 917, respectively. Recombinant His-tagged MPK6 was activated and then used to phosphorylate His-tagged NIA2c and NIA2d in the presence of [γ-32P]ATP. After electrophoresis, the phosphorylated proteins were visualized by autoradiography. Reactions with various components omitted (−) were used as controls. Immunoblotting with anti-Flag and anti-His antibodies was performed to show the loading of Flag-tagged MKK5DD and His-tagged MPK6, NIA2c, and NIA2d, respectively. (C) Activated MPK6 could not phosphorylate NIA1 in the C-terminal region. The assay was performed as described in (A). (D) Treatment with DEX enhanced the phosphorylation of MPK6 in MKK5DD mutants. Protein extracts from MKK5DD and MKK5DD/mpk6-3 seedlings were treated with 2 μM DEX for the indicated times, and 10 μg protein extract was used for SDS-PAGE. Activated MPK6 and MPK3 were detected by immunoblotting using the phospho-p44/42 MAPK antibody. (E) Phosphorylation of NIA2 by MPK6 increases NR activity. MKK5DD and MKK5DD/mpk6-3 seedlings were treated with DEX (2 mM). The NR activity in each mutant was determined at the indicated time point. NR activity was measured as described in Figure 2B. Each value represents the mean (±
sd
) of three independent experiments. (F) MPK6 is required for full activation of NR and NO generation. The NR activity (top panel) and NO production (middle panel) in 35S-NIA2 and mpk6-3/35S-NIA2 transgenic plants were measured as described in Figure 2. Each value represents the mean (±
sd
) of three independent experiments. The NR activity and NO production in the wild type were set as 100%. Protein extract (10 μg) from 35S-NIA2 and mpk6-3/35S-NIA2 transgenic plants was separated by electrophoresis, and immunoblotting with anti-Flag antibody was performed to determine the amount of Flag-tagged NIA2 proteins (bottom panel).
Figure 6.
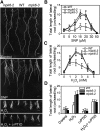
Changes in Morphology of the Root System in Wild-Type and mpk6-3 Plants in Response to NO and H2O2. (A) The number and length of lateral roots was greater in mpk6 mutant plants than in wild-type plants after treatment with SNP or H2O2. Five-day-old seedlings of the wild type, mpk6-2, and mpk6-3 were transferred to vertical MS plates containing (top to bottom): no additions (control); 15 μM SNP, 2.5 mM H2O2, or 2.5 mM H2O2 plus 0.5 mM c-PTIO. The photographs were taken at 10 d (top two panels) or 15 d (bottom two panels) after transfer. Bar = 0.5 cm. (B) Average length of the lateral roots of all plants on MS medium supplemented with SNP at the indicated concentrations. Data represent means (±
sd
) of three independent experiments (~20 plants per point). (C) Average length of the lateral roots of each type of plant on MS medium supplemented with H2O2 at the indicated concentrations. Data represent means (±
sd
) of three independent experiments (at least 30 plants). (D) c-PTIO treatment abolished the H2O2-induced differences in lateral roots between the wild type and mpk6 mutant. Five-day-old seedlings of the wild type, mpk6-2, and mpk6-3 were transferred to MS plates (control) or vertical MS plates that contained 2.5 mM H2O2 and 0.5 mM c-PTIO. Data represent means (±
sd
) of three independent experiments (at least 30 plants).
Figure 7.
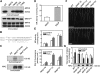
Ser-627 of NIA2 Was a Putative Site for MPK6 Phosphorylation and Essential for the Efficient Activation of NR and Development of Lateral Roots. (A) Ser-627 in the hinge 2 domain of NIA2 is the MPK6 phosphorylation site. Recombinant His-tagged MPK6 was activated and then used to phosphorylate His-tagged NIA2d protein with either the S627A, T649A, T715A, or the T799A mutation in the presence of [γ-32P]ATP. After electrophoresis, the phosphorylated proteins were visualized by autoradiography. Immunoblotting with anti-Flag and anti-His antibodies was performed to show the loading of Flag-tagged MKK5DD and His-tagged MPK6 and NIA2d, respectively. (B) Sequence alignment shows three Ser residues adjacent to the phosphorylation site are missing in NIA1 protein. The first amino acid in each sequence used for alignment is indicated, and the phosphorylation site is underlined. (C) Phosphorylation of native NIA2 protein by MPK6. Flag-tagged full-length NIA2WT and NIA2S627A protein were immunoprecipitated from DEX-treated NIA2WT and NIA2A transgenic plants and were used for the phosphorylation assay as described in (A). Only wild-type NIA2 protein could be phosphorylated by MPK6. Immunoblotting with anti-Flag antibodies was performed to show the loading of NIA2 protein. (D) NR activity was stimulated by the phosphorylation of NIA2-Ser627. Five micrograms of purified NIA2A and NIA2D protein from P. pastoris was used for NR activity assay as described in Figure 2B. Each value represents the mean (±
sd
) of three independent experiments. (E) Phosphorylation and substitution of Ser-627 of NIA2 affected NR activity and NO production. The NR activity (top panel) and NO production (bottom panel) in NIA2A, NIA2WT, and NIA2D transgenic plants treated with 10 μM H2O2 or a water control were measured as described in Figure 2B. Each value represents the mean (±
sd
) of three independent experiments. (F) Photography of transgenic plants containing the modifications of Ser-627 of NIA2 on an MS agar plate (control, top) and an MS agar plate supplemented with 15 μM SNP (bottom). Five-day-old seedlings of the wild-type, NIA2A, NIA2WT, and NIA2D transgenic plants were transferred to the plate. The photographs were taken 10 d after the transfer. Bar = 0.5 cm. (G) Average of the total length of lateral roots of wild-type and transgenic plants on MS medium (control) or MS supplemented with 15 μM SNP. Data represent means (±
sd
) of three independent experiments (~20 plants per point).
Figure 8.
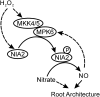
Model for the Putative Pathway of NO Biosynthesis and Signal Transduction Mediated by H2O2-Activated MPK6 in Arabidopsis. The solid and dotted arrows indicate the positive regulation based on our data and the previous results, respectively. Please see the text for a detailed description of this model.
Similar articles
- AIK1, A Mitogen-Activated Protein Kinase, Modulates Abscisic Acid Responses through the MKK5-MPK6 Kinase Cascade.
Li K, Yang F, Zhang G, Song S, Li Y, Ren D, Miao Y, Song CP. Li K, et al. Plant Physiol. 2017 Feb;173(2):1391-1408. doi: 10.1104/pp.16.01386. Epub 2016 Dec 2. Plant Physiol. 2017. PMID: 27913741 Free PMC article. - Dual roles of the MPK3 and MPK6 mitogen-activated protein kinases in regulating Arabidopsis stomatal development.
Wu M, Wang S, Ma P, Li B, Hu H, Wang Z, Qiu Q, Qiao Y, Niu D, Lukowitz W, Zhang S, Zhang M. Wu M, et al. Plant Cell. 2024 Oct 3;36(10):4576-4593. doi: 10.1093/plcell/koae225. Plant Cell. 2024. PMID: 39102898 - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Platelet-rich therapies for musculoskeletal soft tissue injuries.
Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Moraes VY, et al. Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD010071. doi: 10.1002/14651858.CD010071.pub3. Cochrane Database Syst Rev. 2014. PMID: 24782334 Free PMC article. Review.
Cited by
- Deciphering systemic wound responses of the pumpkin extrafascicular phloem by metabolomics and stable isotope-coded protein labeling.
Gaupels F, Sarioglu H, Beckmann M, Hause B, Spannagl M, Draper J, Lindermayr C, Durner J. Gaupels F, et al. Plant Physiol. 2012 Dec;160(4):2285-99. doi: 10.1104/pp.112.205336. Epub 2012 Oct 19. Plant Physiol. 2012. PMID: 23085839 Free PMC article. - GabiPD - The GABI Primary Database integrates plant proteomic data with gene-centric information.
Usadel B, Schwacke R, Nagel A, Kersten B. Usadel B, et al. Front Plant Sci. 2012 Jul 9;3:154. doi: 10.3389/fpls.2012.00154. eCollection 2012. Front Plant Sci. 2012. PMID: 22787465 Free PMC article. - H2 O2 , NO, and H2 S networks during root development and signalling under physiological and challenging environments: Beneficial or toxic?
Mukherjee S, Corpas FJ. Mukherjee S, et al. Plant Cell Environ. 2023 Mar;46(3):688-717. doi: 10.1111/pce.14531. Epub 2023 Jan 12. Plant Cell Environ. 2023. PMID: 36583401 Free PMC article. Review. - Comparative transcriptomic analysis uncovers conserved pathways involved in adventitious root formation in poplar.
Luo J, Nvsvrot T, Wang N. Luo J, et al. Physiol Mol Biol Plants. 2021 Sep;27(9):1903-1918. doi: 10.1007/s12298-021-01054-7. Epub 2021 Aug 31. Physiol Mol Biol Plants. 2021. PMID: 34629770 Free PMC article. - Nitrosative stress triggers microtubule reorganization in Arabidopsis thaliana.
Lipka E, Müller S. Lipka E, et al. J Exp Bot. 2014 Aug;65(15):4177-89. doi: 10.1093/jxb/eru194. Epub 2014 May 6. J Exp Bot. 2014. PMID: 24803503 Free PMC article.
References
- Ahlfors R., Macioszek V., Rudd J., Brosché M., Schlichting R., Scheel D., Kangasjarvi J. (2004). Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40: 512–522 - PubMed
- Alessi D.R., Cuenda A., Cohen P., Dudley D.T., Saltiel A.R. (1995). PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270: 27489–27494 - PubMed
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.-L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. (2002). MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 - PubMed
- Bergmann D.C., Lukowitz W., Somerville C.R. (2004). Stomatal development and pattern controlled by a MAPKK kinase. Science 304: 1494–1497 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases