Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease - PubMed (original) (raw)
Inhibition of the visual cycle by A2E through direct interaction with RPE65 and implications in Stargardt disease
Gennadiy Moiseyev et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Stargardt disease (STGD) is the major form of inherited juvenile macular degeneration. Pyridinium bis-retinoid A2E is a major component of lipofuscin which accumulates in retinal pigment epithelium (RPE) cells in STGD and contributes to the disease pathogenesis. However, the precise role of A2E in vision loss is unclear. Here we report that A2E efficiently inhibits RPE65 isomerohydrolase, a key enzyme in the visual cycle. Subretinal injection of A2E significantly inhibited retinoid isomerohydrolase activity in mice. Likewise, A2E also inhibited isomerohydrolase activity in cells coexpressing RPE65, lecithin retinol acyltransferase (LRAT), and cellular retinaldehyde-binding protein. In vitro isomerohydrolase activity assays confirmed that A2E inhibited enzymatic activity of recombinant RPE65 in a concentration-dependent manner, but did not inhibit LRAT activity. The inhibition type for isomerohydrolase was found to be reversible and competitive with K(i) = 13.6 μM. To determine the direct interaction of A2E with RPE65 protein, fluorescence binding assays were performed. As shown by fluorimetric titration, binding of purified RPE65 with A2E enhanced the bis-retinoid fluorescence. Consistently, the fluorescence of RPE65 decreased upon incubation with A2E. Both of these experiments suggest a direct, specific binding of A2E to RPE65. The binding constant for A2E and purified RPE65 was calculated to be 250 nM. These results demonstrate that A2E inhibits the regeneration of 11-cis retinal, the chromophore of visual pigments, which represents a unique mechanism by which A2E may impair vision in STGD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
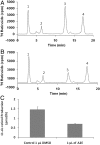
Fig. 1.
Inhibition of the isomerohydrolase activity by A2E in mouse eyes. BALB/c mice received a subretinal injection of 1 μL of 30 mM of A2E dissolved in DMSO or 1 μL of DMSO alone as control. Twenty-four hours after the injection, eyes were enucleated, and the eyecups homogenized in the grinder and incubated with 0.2 μM of all-trans [3H]-retinol for 2 h at 37 °C. The retinoids generated were analyzed by HPLC. (A) HPLC elution profile for the mice injected by 1 μL DMSO; (B) HPLC elution profile for the mice injected by 1 μL A2E dissolved in DMSO. (C) Amounts of 11-cis retinol generated were quantified based on the standard and averaged (mean ± SD, n = 4). Peaks were identified by coelution with corresponding retinoid standards. Peak 1, retinyl esters; 2, all-trans retinal; 3, 11-cis retinol; 4, all-trans retinol.
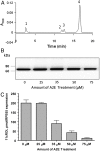
Fig. 2.
Inhibition of the isomerohydrolase activity by A2E in cell culture. The 293A-LRAT cells infected by Ad-RPE65 at MOI 100 were preincubated for 6 h with the indicated concentrations of A2E followed by the addition of 2 μM of all-trans retinol and another 2 h incubation. At approximately 24 h after infection, the cells were harvested, and retinoids extracted with methanol and hexane and saponified. Production of 11-cis retinol was monitored by a normal phase HPLC. (A) HPLC elution profile. Peak 1, retinyl esters; 2, 11-cis retinol; 3, 13-cis retinol; 4, all-trans retinol. (B) Western blot analysis with an antibody specific for RPE65. Equal amounts of total cell lysate were loaded in each lane. (C) Dependence of the amount of generated 11-cis retinol on A2E concentration in culture medium (mean ± SD, n = 4).
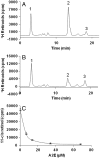
Fig. 3.
Inhibition of the isomerohydrolase activity by A2E in vitro. The same amount (62 μg) of total proteins from 293A-LRAT cells infected by Ad-RPE65 at MOI 100 was incubated with 0.2 μM of all-trans [3H]-retinol in the presence or absence of A2E for 2 h at 37 °C. The retinoids generated were analyzed by HPLC. (A) HPLC elution profile without A2E; (B) with 13.5 μM A2E. Peak 1, retinyl esters; 2, 11-cis retinol; 3, all-trans retinol. (C) A2E concentration-dependent inhibition of 11-cis retinol generation (mean ± SD, n = 4).
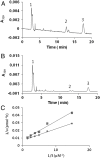
Fig. 4.
Competitive inhibition of RPE65 isomerohydrolase by A2E in a liposome-based isomerohydrolase assay. All-trans retinyl ester incorporated in liposomes was used as a substrate for RPE65 expressed in 293A cells infected by Ad-RPE65 at MOI 100. (A) HPLC elution profile without A2E; (B) with 6.8 μM A2E. Peak 1, retinyl esters; 2, 11-cis retinol; 3, all-trans retinol. (C) Lineweaver–Burk plot of 11-cis retinol generation by RPE65. Liposomes with increasing concentrations (S) of all-trans retinyl palmitate were incubated with equal amounts (25 μg) of purified chicken recombinant RPE65 in the absence (♦) or presence (▪) of A2E (6.7 μM).
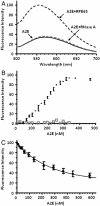
Fig. 5.
Fluorescence measurement of binding of A2E to RPE65. (A) Binding of A2E to purified chicken RPE65. Fluorescence emission spectra of 1 μM A2E in PBS, 0.1% CHAPS or after the addition of 1 μM RPE65 or 1 μM RNase A (control). Excitation peaked at 400 nm. (B) Titration of chicken RPE65 with A2E as measured by the increase in fluorescence intensity of A2E. Excitation was recorded at wavelength 400 nm, emission at wavelength 576 nm. The titration system consisted of 2 ml of 0.1 μM of RPE65 (●) or 0.1 μM pancreatic ribonuclease A (□) in 0.1% CHAPS, PBS buffer, pH 7.4. (C) Titration of chicken RPE65 with A2E as measured by the quenching of protein fluorescence. Excitation was recorded at wavelength 278 nm, emission at wavelength 340 nm. The titration system consisted of 2 mL of 0.1 μM of RPE65 in 0.1% CHAPS in PBS, pH 7.4 (mean ± SD, n = 4).
Similar articles
- The novel visual cycle inhibitor (±)-RPE65-61 protects retinal photoreceptors from light-induced degeneration.
Wang Y, Ma X, Muthuraman P, Raja A, Jayaraman A, Petrukhin K, Cioffi CL, Ma JX, Moiseyev G. Wang Y, et al. PLoS One. 2022 Oct 13;17(10):e0269437. doi: 10.1371/journal.pone.0269437. eCollection 2022. PLoS One. 2022. PMID: 36227868 Free PMC article. - The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model.
Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. Kim SR, et al. Proc Natl Acad Sci U S A. 2007 Dec 4;104(49):19273-8. doi: 10.1073/pnas.0708714104. Epub 2007 Nov 28. Proc Natl Acad Sci U S A. 2007. PMID: 18048333 Free PMC article. - Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E.
Kim SR, Fishkin N, Kong J, Nakanishi K, Allikmets R, Sparrow JR. Kim SR, et al. Proc Natl Acad Sci U S A. 2004 Aug 10;101(32):11668-72. doi: 10.1073/pnas.0403499101. Epub 2004 Jul 26. Proc Natl Acad Sci U S A. 2004. PMID: 15277666 Free PMC article. - Retinal pigment epithelium 65 kDa protein (RPE65): An update.
Kiser PD. Kiser PD. Prog Retin Eye Res. 2022 May;88:101013. doi: 10.1016/j.preteyeres.2021.101013. Epub 2021 Oct 2. Prog Retin Eye Res. 2022. PMID: 34607013 Free PMC article. Review. - A2E, a byproduct of the visual cycle.
Sparrow JR, Fishkin N, Zhou J, Cai B, Jang YP, Krane S, Itagaki Y, Nakanishi K. Sparrow JR, et al. Vision Res. 2003 Dec;43(28):2983-90. doi: 10.1016/s0042-6989(03)00475-9. Vision Res. 2003. PMID: 14611934 Review.
Cited by
- Allelic and phenotypic heterogeneity in ABCA4 mutations.
Burke TR, Tsang SH. Burke TR, et al. Ophthalmic Genet. 2011 Sep;32(3):165-74. doi: 10.3109/13816810.2011.565397. Epub 2011 Apr 21. Ophthalmic Genet. 2011. PMID: 21510770 Free PMC article. Review. - Membrane receptors and transporters involved in the function and transport of vitamin A and its derivatives.
Sun H. Sun H. Biochim Biophys Acta. 2012 Jan;1821(1):99-112. doi: 10.1016/j.bbalip.2011.06.010. Epub 2011 Jun 17. Biochim Biophys Acta. 2012. PMID: 21704730 Free PMC article. Review. - Vitamin A cycle byproducts explain retinal damage and molecular changes thought to initiate retinal degeneration.
Zhang D, Mihai DM, Washington I. Zhang D, et al. Biol Open. 2021 Nov 15;10(11):bio058600. doi: 10.1242/bio.058600. Epub 2021 Nov 29. Biol Open. 2021. PMID: 34842275 Free PMC article. - Genetic determinants of macular pigments in women of the Carotenoids in Age-Related Eye Disease Study.
Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, Liu Z, Igo RP Jr, Truitt B, Klein ML, Snodderly DM, Blodi BA, Gehrs KM, Sarto GE, Wallace RB, Robinson J, LeBlanc ES, Hageman G, Tinker L, Mares JA. Meyers KJ, et al. Invest Ophthalmol Vis Sci. 2013 Mar 28;54(3):2333-45. doi: 10.1167/iovs.12-10867. Invest Ophthalmol Vis Sci. 2013. PMID: 23404124 Free PMC article. - Morphological and physiological retinal degeneration induced by intravenous delivery of vitamin A dimers in rabbits.
Penn J, Mihai DM, Washington I. Penn J, et al. Dis Model Mech. 2015 Feb;8(2):131-8. doi: 10.1242/dmm.017194. Epub 2014 Dec 12. Dis Model Mech. 2015. PMID: 25504631 Free PMC article.
References
- Stargardt K. Über familiäre, progressive Degeneration in der Maculagegend des Auges. Graef Arch Clin Exp. 1909;71:534–550.
- Blacharski PA. Fundu flavimaculatus. In: Newsome DA, editor. Retinal Dystrophies and Degenerations. New York: Raven; 1988. pp. 135–159.
- Fishman GA, Farber M, Patel BS, Derlacki DJ. Visual acuity loss in patients with Stargardt’s macular dystrophy. Ophthalmology. 1987;94:809–814. - PubMed
- Noble KG, Carr RE. Stargardt’s disease and fundus flavimaculatus. Arch Ophthalmol. 1979;97:1281–1285. - PubMed
- Allikmets R, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY019309/EY/NEI NIH HHS/United States
- P20 RR024215/RR/NCRR NIH HHS/United States
- R01 EY019309/EY/NEI NIH HHS/United States
- EY012231/EY/NEI NIH HHS/United States
- P20RR024215/RR/NCRR NIH HHS/United States
- R01 EY018659/EY/NEI NIH HHS/United States
- EY018659/EY/NEI NIH HHS/United States
- R01 EY012231/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous