Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration - PubMed (original) (raw)
. 2010 Sep 21;8(9):e1000479.
doi: 10.1371/journal.pbio.1000479.
Henrike Grund, Kirstin Wingler, Melanie E Armitage, Emma Jones, Manish Mittal, David Barit, Tobias Schwarz, Christian Geis, Peter Kraft, Konstanze Barthel, Michael K Schuhmann, Alexander M Herrmann, Sven G Meuth, Guido Stoll, Sabine Meurer, Anja Schrewe, Lore Becker, Valérie Gailus-Durner, Helmut Fuchs, Thomas Klopstock, Martin Hrabé de Angelis, Karin Jandeleit-Dahm, Ajay M Shah, Norbert Weissmann, Harald H H W Schmidt
Affiliations
- PMID: 20877715
- PMCID: PMC2943442
- DOI: 10.1371/journal.pbio.1000479
Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration
Christoph Kleinschnitz et al. PLoS Biol. 2010.
Abstract
Ischemic stroke is the second leading cause of death worldwide. Only one moderately effective therapy exists, albeit with contraindications that exclude 90% of the patients. This medical need contrasts with a high failure rate of more than 1,000 pre-clinical drug candidates for stroke therapies. Thus, there is a need for translatable mechanisms of neuroprotection and more rigid thresholds of relevance in pre-clinical stroke models. One such candidate mechanism is oxidative stress. However, antioxidant approaches have failed in clinical trials, and the significant sources of oxidative stress in stroke are unknown. We here identify NADPH oxidase type 4 (NOX4) as a major source of oxidative stress and an effective therapeutic target in acute stroke. Upon ischemia, NOX4 was induced in human and mouse brain. Mice deficient in NOX4 (Nox4(-/-)) of either sex, but not those deficient for NOX1 or NOX2, were largely protected from oxidative stress, blood-brain-barrier leakage, and neuronal apoptosis, after both transient and permanent cerebral ischemia. This effect was independent of age, as elderly mice were equally protected. Restoration of oxidative stress reversed the stroke-protective phenotype in Nox4(-/-) mice. Application of the only validated low-molecular-weight pharmacological NADPH oxidase inhibitor, VAS2870, several hours after ischemia was as protective as deleting NOX4. The extent of neuroprotection was exceptional, resulting in significantly improved long-term neurological functions and reduced mortality. NOX4 therefore represents a major source of oxidative stress and novel class of drug target for stroke therapy.
Conflict of interest statement
HHHWS and KW declare a potential competing interest as shareholder and previous employee, respectively, of Vasopharm GmbH, which develops NADPH oxidase inhibitors such as VAS2870. All authors declare that they adhere to all PLoS Biology policies on sharing data and materials as detailed in the PLoS Biology guide for authors.
Figures
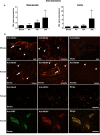
Figure 1. Induction of NOX4 expression after ischemic stroke in mice and humans.
(A) Relative gene expression of Nox4 in the ischemic basal ganglia (left) and cortex (right) of wild-type mice after sham operation and 4 h, 12 h, and 24 h after tMCAO (n = 5). *, p<0.05, one-way ANOVA, Bonferroni post-hoc test, compared with sham-treated controls. (B) Immunohistochemical detection of NOX4 protein in ischemic brains of wild-type mice (after sham operation or tMCAO, day 1) and humans (samples from stoke patients, after routine autopsy). We compared NOX4 immunolabeling in the ischemic forebrain cortex and the unaffected contralateral side. In ischemic samples, NOX4 was predominantly expressed in neurons (arrowheads) and endothelial cells (arrows). This distribution was confirmed by visualization of NOX4 and NeuN or NOX4 and von Willebrand Factor in the same structures. All scale bars represent 100 µm.
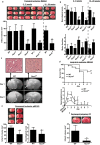
Figure 2. Nox4 deficiency confers long-term neuroprotection and reduces mortality after acute ischemic stroke in young adult and aged mice of either sex.
(A) Upper panel shows representative TTC staining of three corresponding coronal brain sections of 6- to 8-wk-old male and female wild-type (WT) mice, male Nox1 y/− mice, male Nox2 y/− mice, and male and female Nox4 −/− mice, as well as 18- to 20-wk-old male wild-type and Nox4 −/− mice on day 1 after tMCAO. The ischemic infarcts (white) appear smallest in the Nox4 −/− mice of either age or sex (arrows), and this result was confirmed by infarct volumetry (lower panel). ***, p<0.0001, and **, p<0.001, one-way ANOVA, Bonferroni post-hoc test compared with wild-type mice (n = 8–19 per group). (B) Neurological Bederson score (upper panel) and motor score (lower panel) on day 1 after tMCAO in the eight mouse groups indicated above. (C) Serial magnetic resonance images of cerebral infarcts 1 d and 6 d after tMCAO in wild-type and Nox4 −/− mice (lower panel). The broken white lines show hyperintense ischemic lesions on day 1 after tMCAO in wild-type and Nox4 −/− mice. Infarcts on day 1 are smaller in Nox4 −/− mice than in wild-type mice and remain restricted to the basal ganglia on day 6. Hematoxylin and eosin staining confirmed neuronal damage in the cortex of wild-type mice 24 h after tMCAO (top panel, left), whereas cortical integrity was preserved in Nox4 −/− mice (top panel, right). (D) Mortality (upper panel) and long-term functional outcome (Bederson score, lower panel) in 6- to 8-wk-old male Nox4 −/− mice and wild-type controls. Survival curve (upper panel): **, p = 0.0039, log-rank test compared with wild-type mice (n = 10–15 per group). Long-term outcome (lower panel): ***, p<0.0001, and *, p<0.05, one-way ANOVA, Bonferroni post-hoc test compared with wild-type mice (n = 10–15 per group). (E) Upper panel shows representative TTC staining of three corresponding coronal brain sections of 6- to 8-wk-old male wild-type mice (left) and matching Nox4 −/− mice (right) on day 1 after pMCAO. Lower panel: Infarct volumes as measured by infarct volumetry (left) and Neurological Bederson score (right). Nox4 deficiency also protects the brain from permanent ischemia. **, p<0.001, and *, p<0.05, two-tailed Student's _t_-test compared with wild-type mice (n = 7–11 per group). (F) Representative coronal brain sections of wild-type and Nox4 −/− mice stained with TTC on day 1 after permanent cortical photothrombosis (PT) (upper panel). Cortical infarctions are smaller in the absence of NOX4 (arrow). The lower panel shows infarct volumes in wild-type and Nox4 −/− mice on day 1 after cortical photothrombosis. **, p<0.001, two-tailed Student's _t_-test compared with wild-type mice (n = 7 per group). All scale bars represent 100 µm.
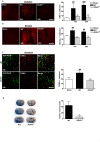
Figure 3. Nox4 deficiency confers neuroprotection by reducing oxidative stress, neuronal apoptosis, and disruption of the blood–brain barrier.
(A and B) Left panels show representative brain sections from sham-operated wild-type (WT) mice and wild-type and Nox4 −/− mice 24 h after tMCAO. Sections were stained for ROS and oxidative chemistry using dihydroethidium (DHE) (A), or stained for reactive nitrogen species by using nitrotyrosine (B). Right panels show the number of cells per square millimeter that are positive for ROS or oxidative stress (A) or reactive nitrogen species (B) in the ischemic hemispheres of sham-operated wild-type mice and wild-type and Nox4 −/− mice 12 h and 24 h after tMCAO (n = 4 per group). (C) Left panels show representative brain sections from sham-operated wild-type mice and wild-type and Nox4 −/− mice 24 h after tMCAO, immunolabeled for the neuronal marker NeuN and subjected to TUNEL to show apoptosis. Right panel shows the number of TUNEL-positive neurons per square millimeter in the ischemic hemispheres of sham-operated wild-type mice and wild-type and Nox4 −/− mice 24 h after tMCAO (n = 4 per group). (D) Left panels show corresponding coronal brain sections of wild-type and Nox4 −/− mice on day 1 after tMCAO and injection of Evans blue. Extravasation of Evans blue was reduced in areas where infarcts were regularly present in Nox4 −/− mice (basal ganglia, broken white line). The right panel shows the extent of extravasation (i.e., edema volume) as determined by planimetry in the wild-type and Nox4 −/− mice 24 h after tMCAO (n = 6 per group). For (A–C), ###, p<0.0001, and ##, p<0.001, compared with sham-treated mice; ***, p<0.0001, and **, p<0.001, compared with wild-type mice by two-way ANOVA, Bonferroni post-hoc test. For (D), **, p<0.001, Two-tailed Student′s _t_-test, compared with wild-type mice. All scale bars represent 100 µm.
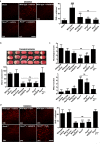
Figure 4. The NADPH oxidase inhibitor VAS2870 protects the brain from damage during acute ischemic stroke.
(A) Left panel shows representative images of vital brain slices from sham-operated wild-type (WT) mice and wild-type mice and Nox4 −/− mice 12 h after tMCAO. Slices were incubated ex vivo with VAS2870 (10 µM) or carrier solution (1% DMSO; control) for 30 min and stained with dihydroethidium (DHE) to detect ROS. Right panel shows number of ROS-positive cells per square millimeter in brain slices from mice in the different treatment groups (n = 5 per group). (B) Upper panel shows representative TTC staining of three corresponding coronal brain sections of wild-type mice treated with (left to right) carrier solution (10% DMSO; control), 100 µg of apocynin intravenously 1 h after tMCAO, or 2 mg of VAS2870 intrathecally 2 h and 12 h after tMCAO, untreated Nox4 −/− mice, Nox4 −/− mice treated with 2 mg of VAS2870 intrathecally 2 h and 12 h after tMCAO, and Nox4 −/− mice treated with H2O2 intrathecally (15 mg/kg) immediately after the occlusion of the middle cerebral artery and then every hour until 6 h after stroke induction. Ischemic infarcts (white) appear smaller (arrows) in VAS2870-treated wild-type mice and Nox4 −/− mice than in control mice, but those in apocynin-treated mice are similar to those in control mice. VAS2870 could not further decrease infarct volumes in Nox4 −/− mice. Exogenous H2O2 reversed the stroke-protective phenotype in Nox4 −/− mice. These results were confirmed by infarct volumetry (lower panel) (n = 7–10 per group). (C) Neurological Bederson score (upper panel) and motor score (lower panel) on day 1 after tMCAO in the different animal groups indicated in (B) (n = 7–10 per group). (D) Left panel shows representative brain sections from the different animal groups indicated in (B) stained for ROS by using dihydroethidium. Right panel shows corresponding number of ROS-positive cells per square millimeter in the ischemic hemispheres (n = 3–5 per group). For (A), ###, p<0.0001, compared with sham-operated mice, ***, p<0.0001 compared with control mice, ns, not significant, one-way ANOVA, Bonferroni post-hoc test. For (B–D), ***, p<0.0001, **, p<0.001, *, p<0.05, ns, not significant, one-way ANOVA, Bonferroni post-hoc test, compared with controls. All scale bars represent 100 µm.
Comment in
- NOX4: a guilty party in stroke damage.
Sedwick C. Sedwick C. PLoS Biol. 2010 Sep 21;8(9):e1000478. doi: 10.1371/journal.pbio.1000478. PLoS Biol. 2010. PMID: 20877473 Free PMC article. No abstract available.
Similar articles
- Neuroprotection after stroke by targeting NOX4 as a source of oxidative stress.
Radermacher KA, Wingler K, Langhauser F, Altenhöfer S, Kleikers P, Hermans JJ, Hrabě de Angelis M, Kleinschnitz C, Schmidt HH. Radermacher KA, et al. Antioxid Redox Signal. 2013 Apr 20;18(12):1418-27. doi: 10.1089/ars.2012.4797. Epub 2012 Oct 16. Antioxid Redox Signal. 2013. PMID: 22937798 Free PMC article. Review. - NADPH oxidase inhibitor improves outcome of mechanical reperfusion by suppressing hemorrhagic transformation.
Tuo YH, Liu Z, Chen JW, Wang QY, Li SL, Li MC, Dai G, Wang JS, Zhang YL, Feng L, Shi ZS. Tuo YH, et al. J Neurointerv Surg. 2017 May;9(5):492-498. doi: 10.1136/neurintsurg-2016-012377. Epub 2016 Apr 13. J Neurointerv Surg. 2017. PMID: 27075483 - Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques.
Xu S, Chamseddine AH, Carrell S, Miller FJ Jr. Xu S, et al. Redox Biol. 2014 Apr 15;2:642-50. doi: 10.1016/j.redox.2014.04.004. eCollection 2014. Redox Biol. 2014. PMID: 24936437 Free PMC article. - Differential effects of NOX4 and NOX1 on immune cell-mediated inflammation in the aortic sinus of diabetic ApoE-/- mice.
Di Marco E, Gray SP, Chew P, Kennedy K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Di Marco E, et al. Clin Sci (Lond). 2016 Aug 1;130(15):1363-74. doi: 10.1042/CS20160249. Epub 2016 May 17. Clin Sci (Lond). 2016. PMID: 27190136 - NADPH oxidases as therapeutic targets in ischemic stroke.
Kahles T, Brandes RP. Kahles T, et al. Cell Mol Life Sci. 2012 Jul;69(14):2345-63. doi: 10.1007/s00018-012-1011-8. Epub 2012 May 23. Cell Mol Life Sci. 2012. PMID: 22618244 Free PMC article. Review.
Cited by
- Vascular cognitive impairment and Alzheimer's disease: role of cerebral hypoperfusion and oxidative stress.
Kim HA, Miller AA, Drummond GR, Thrift AG, Arumugam TV, Phan TG, Srikanth VK, Sobey CG. Kim HA, et al. Naunyn Schmiedebergs Arch Pharmacol. 2012 Oct;385(10):953-9. doi: 10.1007/s00210-012-0790-7. Epub 2012 Aug 8. Naunyn Schmiedebergs Arch Pharmacol. 2012. PMID: 22872171 Review. - Blocking of bradykinin receptor B1 protects from focal closed head injury in mice by reducing axonal damage and astroglia activation.
Albert-Weissenberger C, Stetter C, Meuth SG, Göbel K, Bader M, Sirén AL, Kleinschnitz C. Albert-Weissenberger C, et al. J Cereb Blood Flow Metab. 2012 Sep;32(9):1747-56. doi: 10.1038/jcbfm.2012.62. Epub 2012 May 9. J Cereb Blood Flow Metab. 2012. PMID: 22569191 Free PMC article. - Targeting oxidative stress for the treatment of ischemic stroke: Upstream and downstream therapeutic strategies.
Li W, Yang S. Li W, et al. Brain Circ. 2016 Oct-Dec;2(4):153-163. doi: 10.4103/2394-8108.195279. Epub 2016 Dec 6. Brain Circ. 2016. PMID: 30276293 Free PMC article. Review. - Poldip2 knockdown inhibits vascular smooth muscle proliferation and neointima formation by regulating the expression of PCNA and p21.
Datla SR, L Hilenski L, Seidel-Rogol B, Dikalova AE, Harousseau M, Punkova L, Joseph G, Taylor WR, Lassègue B, Griendling KK. Datla SR, et al. Lab Invest. 2019 Mar;99(3):387-398. doi: 10.1038/s41374-018-0103-y. Epub 2018 Sep 20. Lab Invest. 2019. PMID: 30237457 Free PMC article. - Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke.
Casas AI, Kleikers PW, Geuss E, Langhauser F, Adler T, Busch DH, Gailus-Durner V, de Angelis MH, Egea J, Lopez MG, Kleinschnitz C, Schmidt HH. Casas AI, et al. J Clin Invest. 2019 Mar 18;129(4):1772-1778. doi: 10.1172/JCI124283. eCollection 2019 Mar 18. J Clin Invest. 2019. PMID: 30882367 Free PMC article.
References
- World Health Organization. The top ten causes of death. Fact sheet number 310. 2008. 5 Geneva: World Health Organization. Available: http://www.who.int/mediacentre/factsheets/fs310_2008.pdf.
- Elkins J. S, Johnston S. C. Thirty-year projections for deaths from ischemic stroke in the United States. Stroke. 2003;34:2109–2112. - PubMed
- O'Collins V. E, Macleod M. R, Donnan G. A, Horky L. L, van der Worp B. H, et al. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59:467–477. - PubMed
- Dirnagl U. Bench to bedside: the quest for quality in experimental stroke research. J Cereb Blood Flow Metab. 2006;26:1465–1478. - PubMed
- Whalley K. Slicing into stroke therapeutics. Nat Rev Drug Discov. 2006;5:632–632.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous