Development of stable phosphohistidine analogues - PubMed (original) (raw)
Development of stable phosphohistidine analogues
Jung-Min Kee et al. J Am Chem Soc. 2010.
Abstract
Protein phosphorylation is one of the most common and extensively studied posttranslational modifications (PTMs). Compared to the O-phosphorylation of Ser, Thr, and Tyr residues, our understanding of histidine phosphorylation is relatively limited, particularly in higher eukaryotes, due to technical difficulties stemming from the intrinsic instability and isomerism of phosphohistidine (pHis). We report the design and synthesis of stable and nonisomerizable pHis analogues. These pHis analogues were successfully utilized in solid-phase peptide synthesis and semi-synthesis of histone H4. Significantly, the first antibody that specifically recognizes pHis was obtained using the synthetic peptide as the immunogen.
Figures
Figure 1
(a) Structures of 3- and 1-pHis. (b) Design of pHis analogues. Nitrogen atoms in red (H-bond acceptors) are conserved in the pHis analogues. Hydrolytically labile N—P bonds (red) in pHis are replaced with stable C—P bonds (blue). Both analogues can be prepared from the same building blocks by using different catalysts.
Figure 2
(a) Peptide dot blot using Ab-3pHis. The indicated peptides were labeled with 5-iodoacetamidofluorescein and spotted on a PVDF membrane (50 pmol each). To check for equal loading, the fluorescence of the same membrane was recorded at 520 nm upon excitation at 490 nm. (b) Western blots of recombinant histone H4 and mutants using Ab-3pHis. For the loading control, the initial blot with Ab-3pHis was stripped and reprobed with an α-H4 antibody.
Figure 3
Semi-synthesis of full-length histone H4 containing the 3-pHis analogue. Inset: Western blot showing that Ab-3pHis recognizes pTza-containing H4 protein 21 but not unmodified H4 control.
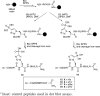
Scheme 1
Synthesis of Protected pTza Isomers
Scheme 2
SPPS of Histone H4 Tail Peptides with the pHis Analogues_a_
Similar articles
- The many ways that nature has exploited the unusual structural and chemical properties of phosphohistidine for use in proteins.
Kalagiri R, Hunter T. Kalagiri R, et al. Biochem J. 2021 Oct 15;478(19):3575-3596. doi: 10.1042/BCJ20210533. Biochem J. 2021. PMID: 34624072 Free PMC article. Review. - A second-generation phosphohistidine analog for production of phosphohistidine antibodies.
Kee JM, Oslund RC, Couvillon AD, Muir TW. Kee JM, et al. Org Lett. 2015 Jan 16;17(2):187-9. doi: 10.1021/ol503320p. Epub 2014 Dec 22. Org Lett. 2015. PMID: 25531910 - Structural basis for differential recognition of phosphohistidine-containing peptides by 1-pHis and 3-pHis monoclonal antibodies.
Kalagiri R, Stanfield RL, Meisenhelder J, La Clair JJ, Fuhs SR, Wilson IA, Hunter T. Kalagiri R, et al. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2010644118. doi: 10.1073/pnas.2010644118. Proc Natl Acad Sci U S A. 2021. PMID: 33547238 Free PMC article. - A journey from phosphotyrosine to phosphohistidine and beyond.
Hunter T. Hunter T. Mol Cell. 2022 Jun 16;82(12):2190-2200. doi: 10.1016/j.molcel.2022.05.007. Epub 2022 Jun 1. Mol Cell. 2022. PMID: 35654043 Free PMC article. Review. - Advances in development of new tools for the study of phosphohistidine.
Makwana MV, Muimo R, Jackson RF. Makwana MV, et al. Lab Invest. 2018 Mar;98(3):291-303. doi: 10.1038/labinvest.2017.126. Epub 2017 Dec 4. Lab Invest. 2018. PMID: 29200202 Review.
Cited by
- Activity-based probe for histidine kinase signaling.
Wilke KE, Francis S, Carlson EE. Wilke KE, et al. J Am Chem Soc. 2012 Jun 6;134(22):9150-3. doi: 10.1021/ja3041702. Epub 2012 May 24. J Am Chem Soc. 2012. PMID: 22606938 Free PMC article. - Tailored therapeutics based on 1,2,3-1_H_-triazoles: a mini review.
Prasher P, Sharma M. Prasher P, et al. Medchemcomm. 2019 May 14;10(8):1302-1328. doi: 10.1039/c9md00218a. eCollection 2019 Aug 1. Medchemcomm. 2019. PMID: 31534652 Free PMC article. Review. - Synthesis of (1,2,3-triazol-4-yl)methyl Phosphinates and (1,2,3-Triazol-4-yl)methyl Phosphates by Copper-Catalyzed Azide-Alkyne Cycloaddition.
Tripolszky A, Németh K, Szabó PT, Bálint E. Tripolszky A, et al. Molecules. 2019 May 31;24(11):2085. doi: 10.3390/molecules24112085. Molecules. 2019. PMID: 31159301 Free PMC article. - Recent Progress on Synthesis of Functionalized 1,5-Disubstituted Triazoles.
Jaiswal MK, Gupta A, Ansari FJ, Pandey VK, Tiwari VK. Jaiswal MK, et al. Curr Org Synth. 2024;21(4):513-558. doi: 10.2174/1570179420666230418123350. Curr Org Synth. 2024. PMID: 38804327 Review. - A new dawn beyond lysine ubiquitination.
Squair DR, Virdee S. Squair DR, et al. Nat Chem Biol. 2022 Aug;18(8):802-811. doi: 10.1038/s41589-022-01088-2. Epub 2022 Jul 27. Nat Chem Biol. 2022. PMID: 35896829 Review.
References
- Walsh CT. Posttranslational Modification of Proteins: Expanding Nature's Inventory. Roberts and Company Publishers; Greenwood Village: 2006. p. 490.
- Druker BJ. Oncologist. 2004;9:357–360. - PubMed
- Cohen P. Nat. Rev. Drug Discovery. 2002;1:309–315. - PubMed
- Khorchid A, Ikura M. Int. J. Biochem. Cell Biol. 2006;38:307–312. - PubMed
- Zetterqvist O, Engstrom L. Biochim. Biophys. Acta. 1966;113:520–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM086868/GM/NIGMS NIH HHS/United States
- R01 GM095880-01/GM/NIGMS NIH HHS/United States
- R37 GM086868/GM/NIGMS NIH HHS/United States
- RC2CA148354/CA/NCI NIH HHS/United States
- GM086868/GM/NIGMS NIH HHS/United States
- RC2 CA148354/CA/NCI NIH HHS/United States
- R01 GM095880/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous