Review: contribution of transgenic models to understanding human prion disease - PubMed (original) (raw)
Review
Review: contribution of transgenic models to understanding human prion disease
J D F Wadsworth et al. Neuropathol Appl Neurobiol. 2010 Dec.
Free PMC article
Abstract
Transgenic mice expressing human prion protein in the absence of endogenous mouse prion protein faithfully replicate human prions. These models reproduce all of the key features of human disease, including long clinically silent incubation periods prior to fatal neurodegeneration with neuropathological phenotypes that mirror human prion strain diversity. Critical contributions to our understanding of human prion disease pathogenesis and aetiology have only been possible through the use of transgenic mice. These models have provided the basis for the conformational selection model of prion transmission barriers and have causally linked bovine spongiform encephalopathy with variant Creutzfeldt-Jakob disease. In the future these models will be essential for evaluating newly identified potentially zoonotic prion strains, for validating effective methods of prion decontamination and for developing effective therapeutic treatments for human prion disease.
© 2010 The Authors. Neuropathology and Applied Neurobiology © 2010 British Neuropathological Society.
Figures
Figure 1
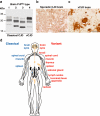
Variant Creutzfeldt-Jakob disease (vCJD) is a distinct human prion strain. (a) Immunoblot of proteinase K digested brain homogenates using antiPrP monoclonal antibody 3F4 showing PrPSc types 1–4 in human brain according to the London classification [90]. Types 1–3 PrPSc are seen in the brain of classical forms of CJD (either sporadic or iatrogenic CJD) and kuru, while type 4 PrPSc is uniquely seen in vCJD brain [25,90,122]. (b,c) Brain sections from sporadic CJD (b) and vCJD (c) showing abnormal PrP accumulation following immunohistochemistry using antiPrP monoclonal antibody ICSM35. Abnormal PrP deposition in sporadic CJD most commonly presents as diffuse, synaptic staining, whereas vCJD is distinguished by the presence of florid PrP plaques consisting of a round amyloid core surrounded by a ring of spongiform vacuoles. Scale bars: 50 µm. (d) Distribution of PrPSc in human tissues. The schematic diagram shows tissues in which PrPSc has been detected using high sensitivity immunoblotting. The vCJD has a peripheral pathogenesis distinct from classical forms of CJD, with a prominent and uniform involvement of lymphoreticular tissues.
Figure 3
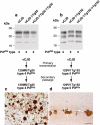
Bovine spongiform encephalopathy (BSE) prions propagate as either variant Creutzfeldt-Jakob disease (vCJD)-like or sporadic CJD (sCJD)-like strains in transgenic mice expressing human prion protein. Primary transmission of BSE prions in transgenic Tg(HuPrP129M+/+Prnp°/°)-35 mice (Tg35) results in the propagation of either type 4 PrPSc and the occurrence of abundant florid PrP plaques that are the neuropathological hallmark of vCJD or type 2 PrPSc and the occurrence of diffuse PrP deposition that is typically seen in sporadic CJD [28]. Molecular and neuropathological characteristics of these distinct prion strains remain stable after secondary passage in the same line of transgenic mice [49]. (a,b) Representative immuno-blots of proteinase-K treated brain homogenates from vCJD and sCJD (PRNP 129 MM genotype with type 2 PrPSc; sCJD T2MM) and transgenic mice analysed with antiPrP monoclonal antibody 3F4. The identity of the brain sample is designated above each lane with the type of PrPSc present in the sample designated below, using the London classification [90]. (c) Representative immunohistochemical analysis of transgenic mouse brain (thalamus) at secondary passage showing abnormal PrP immunoreactivity, including PrP-positive plaques, stained with antiPrP monoclonal antibody ICSM 35. Scale bars: 100 µm.
Figure 2
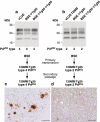
Human prion protein with valine at residue 129 prevents expression of the variant Creutzfeldt-Jakob disease (vCJD) phenotype. Primary and secondary transmission of vCJD prions to transgenic Tg(HuPrP129M+/+Prnp°/°)-35 mice (Tg35) results in faithful propagation of type 4 PrPSc and the occurrence of abundant florid PrP plaques throughout the cortex that are the neuropathological hallmark of vCJD [49]. In contrast, primary transmission of vCJD prions to transgenic Tg(HuPrP129V+/+Prnp°/°)-152 mice (Tg152) produces a novel prion strain that is maintained on secondary passage in the same mice distinguished by the propagation of type 5 PrPSc and a distinct pattern of neuropathology characterized by large nonflorid PrP plaques restricted to the corpus callosum [26,49]. (a,b) Representative immuno-blots of proteinase-K treated brain homogenates from variant CJD and transgenic mice analysed with antiPrP monoclonal antibody 3F4. The identity of the brain sample is designated above each lane with the type of PrPSc present in the sample designated below using the London classification [90]. (c,d) Representative immunohistochemical analysis of transgenic mouse brain at secondary passage showing abnormal PrP plaques stained with antiPrP monoclonal antibodies ICSM 35 (a) or 3F4 (b). Scale bars: 100 µm.
Similar articles
- Guinea Pig Prion Protein Supports Rapid Propagation of Bovine Spongiform Encephalopathy and Variant Creutzfeldt-Jakob Disease Prions.
Watts JC, Giles K, Saltzberg DJ, Dugger BN, Patel S, Oehler A, Bhardwaj S, Sali A, Prusiner SB. Watts JC, et al. J Virol. 2016 Oct 14;90(21):9558-9569. doi: 10.1128/JVI.01106-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27440899 Free PMC article. - Molecular pathology of human prion disease.
Wadsworth JD, Collinge J. Wadsworth JD, et al. Acta Neuropathol. 2011 Jan;121(1):69-77. doi: 10.1007/s00401-010-0735-5. Epub 2010 Aug 8. Acta Neuropathol. 2011. PMID: 20694796 Free PMC article. Review. - Genetic and infectious prion diseases.
Prusiner SB. Prusiner SB. Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review. - The neuropathological phenotype in transgenic mice expressing different prion protein constructs.
DeArmond SJ, Yang SL, Cayetano-Canlas J, Groth D, Prusiner SB. DeArmond SJ, et al. Philos Trans R Soc Lond B Biol Sci. 1994 Mar 29;343(1306):415-23. doi: 10.1098/rstb.1994.0038. Philos Trans R Soc Lond B Biol Sci. 1994. PMID: 7913760 Review. - Nonpathogenic Heterologous Prions Can Interfere with Prion Infection in a Strain-Dependent Manner.
Marín-Moreno A, Aguilar-Calvo P, Pitarch JL, Espinosa JC, Torres JM. Marín-Moreno A, et al. J Virol. 2018 Nov 27;92(24):e01086-18. doi: 10.1128/JVI.01086-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282706 Free PMC article.
Cited by
- Soluble Aβ seeds are potent inducers of cerebral β-amyloid deposition.
Langer F, Eisele YS, Fritschi SK, Staufenbiel M, Walker LC, Jucker M. Langer F, et al. J Neurosci. 2011 Oct 12;31(41):14488-95. doi: 10.1523/JNEUROSCI.3088-11.2011. J Neurosci. 2011. PMID: 21994365 Free PMC article. - Neurotropic influenza A virus infection causes prion protein misfolding into infectious prions in neuroblastoma cells.
Hara H, Chida J, Uchiyama K, Pasiana AD, Takahashi E, Kido H, Sakaguchi S. Hara H, et al. Sci Rep. 2021 May 12;11(1):10109. doi: 10.1038/s41598-021-89586-6. Sci Rep. 2021. PMID: 33980968 Free PMC article. - Structural effects of the highly protective V127 polymorphism on human prion protein.
Hosszu LLP, Conners R, Sangar D, Batchelor M, Sawyer EB, Fisher S, Cliff MJ, Hounslow AM, McAuley K, Leo Brady R, Jackson GS, Bieschke J, Waltho JP, Collinge J. Hosszu LLP, et al. Commun Biol. 2020 Jul 29;3(1):402. doi: 10.1038/s42003-020-01126-6. Commun Biol. 2020. PMID: 32728168 Free PMC article. - Vaccines for prion diseases: a realistic goal?
Napper S, Schatzl HM. Napper S, et al. Cell Tissue Res. 2023 Apr;392(1):367-392. doi: 10.1007/s00441-023-03749-7. Epub 2023 Feb 11. Cell Tissue Res. 2023. PMID: 36764940 Free PMC article. Review. - Strain specific resistance to murine scrapie associated with a naturally occurring human prion protein polymorphism at residue 171.
Striebel JF, Race B, Meade-White KD, LaCasse R, Chesebro B. Striebel JF, et al. PLoS Pathog. 2011 Sep;7(9):e1002275. doi: 10.1371/journal.ppat.1002275. Epub 2011 Sep 29. PLoS Pathog. 2011. PMID: 21980292 Free PMC article.
References
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–50. - PubMed
- Weissmann C. The state of the prion. Nat Rev Microbiol. 2004;2:861–71. - PubMed
- Caughey B, Baron GS. Prions and their partners in crime. Nature. 2006;443:803–10. - PubMed
- Collinge J, Clarke A. A general model of prion strains and their pathogenicity. Science. 2007;318:930–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U123160653/MRC_/Medical Research Council/United Kingdom
- MC_U123160655/MRC_/Medical Research Council/United Kingdom
- MC_U123192748/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources