Protein kinase D-mediated phosphorylation of polycystin-2 (TRPP2) is essential for its effects on cell growth and calcium channel activity - PubMed (original) (raw)
Protein kinase D-mediated phosphorylation of polycystin-2 (TRPP2) is essential for its effects on cell growth and calcium channel activity
Andrew J Streets et al. Mol Biol Cell. 2010.
Abstract
PKD2 is mutated in 15% of patients with autosomal dominant polycystic kidney disease. The PKD2 protein, polycystin-2 or TRPP2, is a nonselective Ca2+-permeable cation channel that has been shown to function at several locations, including primary cilia, basolateral membrane, and at the endoplasmic reticulum (ER). Nevertheless, the factors that regulate the channel activity of polycystin-2 are not well understood. Polycystin-2 has been shown previously to be regulated by phosphorylation at two serine residues (Ser812 and Ser76) with distinct functional consequences. Here, we report the identification of a previously unrecognized phosphorylation site within the polycystin-2 C terminus (Ser801), and we demonstrate that it is phosphorylated by protein kinase D. Phosphorylation at this site was significantly increased in response to serum and epidermal growth factor stimulation. In nonciliated Madin-Darby canine kidney I cells, inducible expression of polycystin-2 inhibited cell proliferation compared with wild-type cells. Mutagenesis at Ser801 abolished these effects and reduced ATP-stimulated Ca2+ release from ER stores. Finally, we show that a pathogenic mutation (S804N) within the consensus kinase recognition sequence abolished Ser801 phosphorylation. These results suggest that growth factor-stimulated, protein kinase D-mediated phosphorylation of polycystin-2 is essential for its ER channel function and links extracellular stimuli to its effects on cell growth and intracellular calcium regulation.
Figures
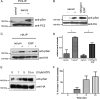
Figure 1.
PC2 is phosphorylated in situ at Ser801. (A) Cell lysates from native mouse kidney cells (M8) and HEK-293 cells transfected with epitope-tagged PC2 (pkTag) were treated with λ-phosphatase for 0 and 60 min and analyzed by Western blotting with PC2- and pkTag-specific antibodies. A clear doublet band is seen in both native and transfected cells (see arrows). The top band disappears after incubation with the enzyme, demonstrating that it is due to phosphorylation. (B) Cell lysates from HEK-293 cells transfected with a C-terminal PKD2 construct were treated with λ-phosphatase for 0 and 90 min and analyzed by Western blotting with PC2-specific antibodies. A clear doublet band is seen. The top band disappears after incubation with the enzyme, demonstrating that it is due to phosphorylation. (C) To confirm the site responsible for the doublet band, single site mutants of CT2 were immunoblotted with a PC2-specific antibody to detect the presence of a phosphorylated banding pattern. Loss of the top phosphorylated band was only seen in cells transfected with the CT2 Ser801 mutant construct and not the Ser812 mutant construct. (D) To confirm the site responsible for the doublet band, single site mutants of PC2 were immunoblotted with an antibody directed to the pkTag epitope tag to detect the presence of a phosphorylated banding pattern. Loss of the top phosphorylated band was only seen in cells transfected with the PC2 Ser801G mutant construct. Conversely, a phosphomimic S801D mutant reduces the electrophoretic mobility of PC2, demonstrating that phosphorylation of Ser801 is sufficient to change the mobility of PC2. (E) Diagram showing the main structural motifs of PC2 relative to the position of previously described phosphorylation sites at Ser76 and Ser812 and the position of the new site at Ser801 reported in this study. (F) Active purified PKC was able to phosphorylate recombinant PC2 C terminus (CT2) in an in vitro kinase assay. The histogram shows that in vitro phosphorylation of the C-terminal of PC2 (CT2) by PKC is reduced by 53% (n = 3) when Ser801 is mutated to Gly (n = 3). Top, autoradiography. Bottom, Coomassie staining showing equal loading of recombinant protein used in the assay. An asterisk indicates a statistically significant difference (p < 0.0065, Student's t test). (G) Two-dimensional gel analysis of phosphorylation of CT2 and CT2 S801G. After an in vitro kinase assay with purified PKC, samples were electrofocused under a pH gradient of 3–10 followed by separation by molecular weight on an 11% SDS-PAGE gel. Proteins were then immunoblotted with an anti-thio antibody. Phosphorylation results in a change in the isoelectric point of CT2. Multiple spots indicate multiple phosphorylation events. Wild-type CT2 separates isoelectrically into three distinct spots. CT2 containing a mutation at Ser801 shows a different pattern of isoelectric separation and has lost the most acidic spot indicating a reduction in overall phosphorylation. (H) A phospho-serine specific antibody (Invitrogen) recognizes HA-PC2 immunoprecipitated from HEK cells. Cell lysates prepared from HA-tagged PC2-expressing HEK cells were immunoprecipitated with an HA antibody. Samples were immunoblotted with an antibody to HA (top) and a phospho-serine antibody (bottom) to detect the presence of a phosphorylated isoform. A clear band was seen with the phospho-serine antibody indicating that PC2 is phosphorylated on serine residues. No signal was seen in mock transfected cells. (I) Immunoblot showing that an anti-phospho-serine antibody (Invitrogen) recognizes the full-length phosphorylated form of PC2 but not serine phosphorylation of the N-terminal domain of PC2 (NT2). (J) To confirm the site responsible for C-terminal phosphorylation, HA-tagged single serine site mutants of PC2 were immunoprecipitated from HEK cell lysates followed by detection with a phospho-serine antibody. Loss of the serine phosphorylated band was only seen in cells transfected with the Ser801 mutant construct. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody.
Figure 2.
Protein kinase D phosphorylates PC2 at Ser801. (A) The consensus PrKD phosphorylation motif (bold residues) and the corresponding motif in human (Q13563), mouse (O35245), and zebrafish (Q2VF27) PC2 are shown with the critical amino acids underlined. (B) To identify the kinase responsible for Ser801 phosphorylation, MDCK cells stably expressing HA-tagged PC2 were treated with specific kinase inhibitors. These included 0.1 μM staurosporine (broad-spectrum kinase inhibitor), 10 μM bisindolylmalemide I (BisI; PKC inhibitor), 10 μM Gö6976 (PKC inhibitor), 20 μM PKC peptide inhibitor (PKC inhibitor), 20 μM PKA peptide inhibitor (PKA inhibitor), and 20 μM KN93 (CAMKII inhibitor). PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Loss of Ser801 phosphorylation was only seen in cells incubated with staurosporine and Gö6976. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (C) MDCK cells stably expressing HA-tagged PC2 were pretreated with DMSO, 10 μM Gö6976, or 10 μM Gö6983 followed by 4-h serum stimulation. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Loss of Ser801 phosphorylation was seen only in cells incubated with Gö6976, suggesting that Ser801 could be phosphorylated by PrKD. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (D) Expression of PrKD increases PC2 phosphorylation at Ser801. Cell lysates prepared from HEK cells coexpressing PrKD with HA-tagged PC2 or PC2 Ser801G were immunoprecipitated with an HA antibody. Samples were immunoblotted with an antibody to PC2 (bottom) and a phospho-serine antibody (top) to detect the presence of Ser801 phosphorylation. A significant increase in phosphorylation was seen when PC2 was coexpressed with PrKD compared with PC2 alone. No signal was seen when PC2 Ser801G was coexpressed with PrKD. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. An asterisk indicates a statistically significant difference (p < 0.0001, Student's t test). (E) HEK cells were treated with control siRNAs or with validated siRNA specific to PrKD as described in Materials and Methods. PrKD knockdown was detected after immunoblotting of cell lysates with a PrKD-specific antibody (Santa Cruz Biotechnology). No reduction was seen in a control protein (calnexin). PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. A significant reduction in Ser801 phosphorylation was only seen in cells treated with PrKD-specific siRNA. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (F) Loss of the Ser801-phosphorylated band was seen both in cells transfected with the Ser801 mutant construct as well as a pathogenic mutant S804N. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody.
Figure 3.
Stimulation of Ser801phosphorylation in kidney epithelial cells. (A) Phosphorylation of endogenous PC2 at Ser801 after 4-h serum stimulation of serum starved M8 cells. PC2 was immunoprecipitated with an antibody directed to the C-terminal (G20) followed by detection with the phospho-serine antibody. The same blots were reprobed with the PC2 mAb 1A11 and show equal loading. (B) Serum and EGF stimulates Ser801 phosphorylation. HEK cells transiently expressing HA-tagged PC2 were serum starved for 24 h followed by stimulation with serum for 4 h or 100 ng/ml EGF for 15 min. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3). Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (C) MDCK cells stably expressing HA-tagged PC2 were serum starved for 24 h followed by 4-h serum stimulation or 100 ng/ml EGF for 15 min. PC2 was immunoprecipitated with an antibody directed to the HA epitope tag, followed by detection with a phospho-serine antibody. (D) Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3). Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. An asterisk indicates a statistically significant difference (p < 0.02, Student's t test). (E) ATP stimulates Ser801 phosphorylation. HEK cells transiently expressing HA-tagged PC2 were serum starved for 24 h followed by stimulation with 10 μM ATP for 0, 1, 5, and 15 min. Equal loading was confirmed by stripping and reprobing the same blot with an HA antibody. (F) Significant increases in Ser801 phosphorylation were seen in stimulated cells (n = 3) after densitometry of Western blots.
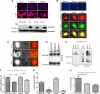
Figure 4.
Effect of Ser801 phosphorylation on the subcellular localization of PC2, protein–protein interactions, and proliferation. (A) Top, immunofluorescence of stably transfected MDCK cells. Expression of HA-tagged PC2 (red) was induced by the addition of 1 μg/ml tetracycline for 24 h. All lines showed clear inducible expression of PC2. Bar, 25 μm). Bottom, Western blotting demonstrates that protein expression of PC2 and Ser801 phosphorylation mutants were equally induced in the presence of 1 μg/ml tetracycline for 24 h. Equal loading was confirmed using calnexin as a control. (B) Immunofluorescence was carried out on 4% paraformaldehyde-fixed MDCK cells. Top, staining for primary cilia with an antibody to acetylated tubulin in cells grown to 5 d postconfluence on filters. MDCK II expressed well-developed primary cilia but MDCK I and MDCK I FRT showed no detectable primary cilia. Bottom, subcellular localization of PC2 in MDCK cells was not altered after mutation of Ser801 to either glycine (phospho-deficient) or aspartic acid (phospho-mimic). PC2 (green) was predominantly localized to the ER where it colocalized with calnexin (red). (C) Immunofluorescence was carried out on 4% paraformaldehyde-fixed MDCK II cells transiently transfected with either wild-type PC2, S801G, or S801D mutants. Staining for primary cilia with an antibody to acetylated tubulin in cells grown to 5 d postconfluence on filters showed that MDCK II expressed well-developed primary cilia. Cilial localization of PC2 in MDCK II cells was not altered after mutation of Ser801 to either glycine (phospho-deficient) or aspartic acid (phospho-mimic). (D) Immunoblot detection with an antibody to HA-PC2 showed that under nonreducing conditions, PC2 forms two higher molecular weight oligomers. Mutation of Ser801 had no effect on PC2 oligomerization. (E) HEK cells were cotransfected with PC1-FLAG and PC2-Ser801G-pkTag. The blots represent protein immunoprecipitated from 0.5 mg of transient transfected HEK-293 total cell lysate by epitope-tagged (FLAG, or Pk) antibodies. Immunoblot detection with 7e12 (for PC1) or pkTag (for PC2) shows that polycystin complex formation was not disrupted by the S801G mutation. (F) The proliferation rate of mock transfected MDCK cells or cells expressing PC2 or a Ser801 phospho-deficient or phospho-mimic mutation was compared using a BrdU ELISA assay (Roche Diagnostics). There was a significant decrease in proliferation in cells expressing PC2 (n = 9). An asterisk indicates a statistically significant difference (p < 0.02). (G) The percentage increase in cells entering S/G2 phase of the cell cycle was significantly reduced after 24-h serum stimulation in cells expressing both PC2 and a Ser801 phospho-mimic mutation (n = 9). An asterisk indicates a statistically significant difference (p < 0.05). (H) The percentage increase in cells entering S/G2 phase of the cell cycle was significantly increased after knockdown of PKD2 in MDCK cells compared with control cells (n = 6). An asterisk indicates a statistically significant difference (p < 0.05).
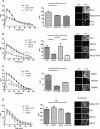
Figure 5.
Ser801 phosphorylation regulates the function of ATP-stimulated PC2-mediated ER Ca2+-release channels. (A) Representative traces of Ca2+ transients elicited by ATP (10 μM) in control MDCK cells (MDCK FRT) and MDCK cells overexpressing PC2 with or without overnight incubation with 1 μg/ml tetracycline shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/_F_0) (left). The area under the curve (AUC) (middle) of ATP-induced Ca2+ transients was measured in each cell line. Bars represent mean ± SEM for 50 cells. PC2-overexpressing cells differed significantly compared with mock or uninduced cells. The increases in Fluo-4 fluorescence (right) can be seen after ATP stimulation. MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (B) Representative traces of Ca2+ transients elicited by ATP (10 μM) in control MDCK cells (MDCK FRT), and MDCK cells overexpressing either PC2, S801G, or S801D shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/_F_0) (left). The AUC (middle) of ATP-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. PC2- and S801D-overexpressing cells differed significantly compared with control and S801G cells (p < 0.05). MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (C) Representative traces of Ca2+ transients elicited by ATP (10 μM) in MDCK cells overexpressing PC2 after 2-h pretreatment with PKC inhibitors (10 μM Gö6976/Gö6983) shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/_F_0) (left). The AUC (middle) of ATP-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. Treatment of cells with Gö6976 significantly reduced the Ca2+ transients elicited by ATP compared with control (DMSO-treated) cells. PC2-expressing cells treated with G06983 showed a much smaller decrease in Ca2+-dependent Fluo-4 fluorescence. The increases in Fluo-4 fluorescence (right) can be seen after ATP stimulation. MDCK cells were loaded with the Fluo-4 dye. All cells respond to ATP stimulus (10 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right). *p < 0.05. (D) Representative traces of Ca2+ transients elicited by ionomycin (1 μm) in control MDCK cells (MDCK FRT), and MDCK cells overexpressing either PC2, S801G, or S801D shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/_F_0) (left). The AUC (middle) of ionomycin-induced Ca2+ transients measured in each cell line. Bars represent mean ± SEM for 50 cells. There was no significant difference between the cell lines. MDCK cells were loaded with the Fluo-4 dye. All cells responded to ionomycin stimulus (1 μM, applied at 0 s) with an increase in intracellular calcium, visualized by an increase in bright pixels from the fluorescence of the indicator dye at 20 s (right).
Figure 6.
PKD2 knockdown reduces ATP-stimulated PC2 mediated ER Ca2+-release. (A) MDCK I cells were treated with control or PKD2 shRNA as described in Materials and Methods. PKD2 knockdown was detected after immunoblotting of cell lysates with a PC2-specific antibody. Calnexin was used as a loading control. (B) Significant decreases in PC2 expression were seen in PKD2 shRN-transfected cells after densitometry of western blots (n = 3). (C) Representative traces of Ca2+ transients elicited by ATP (10 μM) in MDCK I cells transfected with PKD2 or control shRNA is shown as the ratio of Ca2+-dependent Fluo-4 fluorescence over prestimulus background fluorescence (F/_F_0). (D) The area under the curve (AUC) of ATP-induced Ca2+ transients was measured in cell lines transfected with PKD2 or control shRNA. Bars represent mean ± SEM for 50 cells. Cells expressing PKD2 shRNA differed significantly from control cells.
Similar articles
- TRPP2 dysfunction decreases ATP-evoked calcium, induces cell aggregation and stimulates proliferation in T lymphocytes.
Magistroni R, Mangolini A, Guzzo S, Testa F, Rapanà MR, Mignani R, Russo G, di Virgilio F, Aguiari G. Magistroni R, et al. BMC Nephrol. 2019 Sep 13;20(1):355. doi: 10.1186/s12882-019-1540-6. BMC Nephrol. 2019. PMID: 31514750 Free PMC article. - A single amino acid residue constitutes the third dimerization domain essential for the assembly and function of the tetrameric polycystin-2 (TRPP2) channel.
Feng S, Rodat-Despoix L, Delmas P, Ong AC. Feng S, et al. J Biol Chem. 2011 May 27;286(21):18994-9000. doi: 10.1074/jbc.M110.192286. Epub 2011 Apr 7. J Biol Chem. 2011. PMID: 21474446 Free PMC article. - Channel Function of Polycystin-2 in the Endoplasmic Reticulum Protects against Autosomal Dominant Polycystic Kidney Disease.
Padhy B, Xie J, Wang R, Lin F, Huang CL. Padhy B, et al. J Am Soc Nephrol. 2022 Aug;33(8):1501-1516. doi: 10.1681/ASN.2022010053. Epub 2022 Jul 14. J Am Soc Nephrol. 2022. PMID: 35835458 Free PMC article. - Polycystin-2 (TRPP2): Ion channel properties and regulation.
Cantero MDR, Cantiello HF. Cantero MDR, et al. Gene. 2022 Jun 15;827:146313. doi: 10.1016/j.gene.2022.146313. Epub 2022 Mar 18. Gene. 2022. PMID: 35314260 Review. - TRPP2 ion channels: The roles in various subcellular locations.
Tian PF, Sun MM, Hu XY, Du J, He W. Tian PF, et al. Biochimie. 2022 Oct;201:116-127. doi: 10.1016/j.biochi.2022.06.010. Epub 2022 Jun 26. Biochimie. 2022. PMID: 35760123 Review.
Cited by
- Polycystins as components of large multiprotein complexes of polycystin interactors.
Hardy E, Tsiokas L. Hardy E, et al. Cell Signal. 2020 Aug;72:109640. doi: 10.1016/j.cellsig.2020.109640. Epub 2020 Apr 17. Cell Signal. 2020. PMID: 32305669 Free PMC article. Review. - Identification of polycystin 2 missense mutants targeted for endoplasmic reticulum-associated degradation.
Guerriero CJ, Carattino MD, Sharp KG, Kantz LJ, Gresko NP, Caplan MJ, Brodsky JL. Guerriero CJ, et al. Am J Physiol Cell Physiol. 2025 Feb 1;328(2):C483-C499. doi: 10.1152/ajpcell.00776.2024. Epub 2024 Dec 23. Am J Physiol Cell Physiol. 2025. PMID: 39714991 Free PMC article. - Intracellular calcium release modulates polycystin-2 trafficking.
Miyakawa A, Ibarra C, Malmersjö S, Aperia A, Wiklund P, Uhlén P. Miyakawa A, et al. BMC Nephrol. 2013 Feb 11;14:34. doi: 10.1186/1471-2369-14-34. BMC Nephrol. 2013. PMID: 23398808 Free PMC article. - TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches.
Marini M, Titiz M, Souza Monteiro de Araújo D, Geppetti P, Nassini R, De Logu F. Marini M, et al. Biomolecules. 2023 Oct 22;13(10):1557. doi: 10.3390/biom13101557. Biomolecules. 2023. PMID: 37892239 Free PMC article. Review. - Hyperphosphorylation of polycystin-2 at a critical residue in disease reveals an essential role for polycystin-1-regulated dephosphorylation.
Streets AJ, Wessely O, Peters DJ, Ong AC. Streets AJ, et al. Hum Mol Genet. 2013 May 15;22(10):1924-39. doi: 10.1093/hmg/ddt031. Epub 2013 Feb 5. Hum Mol Genet. 2013. PMID: 23390129 Free PMC article.
References
- Ahmmed G. U., Mehta D., Vogel S., Holinstat M., Paria B. C., Tiruppathi C., Malik A. B. Protein kinase Cα phosphorylates the TRPC1 channel and regulates store-operated Ca2+ entry in endothelial cells. J. Biol. Chem. 2004;279:20941–20949. - PubMed
- Barker G., Simmons N. L. Identification of two strains of cultured canine renal epithelial cells (MDCK cells) which display entirely different physiological properties. Q. J. Exp. Physiol. 1981;66:61–72. - PubMed
- Brandlin I., et al. Protein kinase C (PKC)η-mediated PKCμ activation modulates ERK and JNK signal pathways. J. Biol. Chem. 2002;277:6490–6496. - PubMed
- Cai Y., et al. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 2004;279:19987–19995. - PubMed
- Cai Y., Maeda Y., Cedzich A., Torres V. E., Wu G., Hayashi T., Mochizuki T., Park J. H., Witzgall R., Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J. Biol. Chem. 1999;274:28557–28565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous