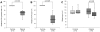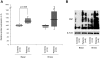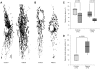Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts - PubMed (original) (raw)
. 2010 Sep 27;5(9):e12962.
doi: 10.1371/journal.pone.0012962.
Lisa Voges, Aleksandar Rakovic, Meike Kasten, Himesha Vandebona, Claudia Hemmelmann, Katja Lohmann, Slobodanka Orolicki, Alfredo Ramirez, Anthony H V Schapira, Peter P Pramstaller, Carolyn M Sue, Christine Klein
Affiliations
- PMID: 20885945
- PMCID: PMC2946349
- DOI: 10.1371/journal.pone.0012962
Mutant Parkin impairs mitochondrial function and morphology in human fibroblasts
Anne Grünewald et al. PLoS One. 2010.
Abstract
Background: Mutations in Parkin are the most common cause of autosomal recessive Parkinson disease (PD). The mitochondrially localized E3 ubiquitin-protein ligase Parkin has been reported to be involved in respiratory chain function and mitochondrial dynamics. More recent publications also described a link between Parkin and mitophagy.
Methodology/principal findings: In this study, we investigated the impact of Parkin mutations on mitochondrial function and morphology in a human cellular model. Fibroblasts were obtained from three members of an Italian PD family with two mutations in Parkin (homozygous c.1072delT, homozygous delEx7, compound-heterozygous c.1072delT/delEx7), as well as from two relatives without mutations. Furthermore, three unrelated compound-heterozygous patients (delEx3-4/duplEx7-12, delEx4/c.924C>T and delEx1/c.924C>T) and three unrelated age-matched controls were included. Fibroblasts were cultured under basal or paraquat-induced oxidative stress conditions. ATP synthesis rates and cellular levels were detected luminometrically. Activities of complexes I-IV and citrate synthase were measured spectrophotometrically in mitochondrial preparations or cell lysates. The mitochondrial membrane potential was measured with 5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolylcarbocyanine iodide. Oxidative stress levels were investigated with the OxyBlot technique. The mitochondrial network was investigated immunocytochemically and the degree of branching was determined with image processing methods. We observed a decrease in the production and overall concentration of ATP coinciding with increased mitochondrial mass in Parkin-mutant fibroblasts. After an oxidative insult, the membrane potential decreased in patient cells but not in controls. We further determined higher levels of oxidized proteins in the mutants both under basal and stress conditions. The degree of mitochondrial network branching was comparable in mutants and controls under basal conditions and decreased to a similar extent under paraquat-induced stress.
Conclusions: Our results indicate that Parkin mutations cause abnormal mitochondrial function and morphology in non-neuronal human cells.
Conflict of interest statement
Competing Interests: Dr. Schapira is a board member of the Royal Free Hospital trust, served as a consultant for BI, GSK, Teva-Lundbeck, Orion-Novartis and EMD Serono, and received research support from the Kattan Trust. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
Figure 1. Mitochondrial function.
(A) Basal ATP synthesis rates. The assay demonstrated a significant reduction in ATP production in the _Parkin_-mutant patients (median [IQR]: 39% [23%, 55%]) compared to controls (set to 100%). (B) Basal ATP levels. Quantifying the overall cellular ATP concentration showed significantly lower levels in the mutants (median [IQR]: 69% [58%, 75%]) than in controls (set to 100%). (C) Mitochondrial membrane potential under basal and oxidative stress conditions. Control (median [IQR]: 100% [94%, 115%]) and patient fibroblasts (median [IQR]: 113% [93%, 128%]) with Parkin mutations show similar membrane potential under basal conditions. When the cells were treated with paraquat (shaded boxes), no relevant changes were detected in the controls (median [IQR]: 102% [100%, 118%]). In the Parkin mutants, a significant drop of the membrane potential was observed (median [IQR]: 84% [81%, 103%]). The median, the interquartile range (IQR), the minimum and the maximum value of 6 (A) or 4 (B) independent experimental runs are plotted. In each experimental run the average ATP level in the controls was set to 100%.
Figure 2. Protein oxidation under basal and stress conditions.
Oxidation of proteins in _Parkin_-mutant fibroblasts and controls was determined by means of an OxyBlot. (A) When quantifying the protein oxidation in each individual using an antibody against DNP, the Parkin mutants (median [IQR]: 123% [113%, 136%]) showed significantly higher levels of oxidation than the controls (median [IQR]: 100% [97%, 105%]). After treatment of the cells with paraquat (shaded boxes), the difference in oxidation between mutants (median [IQR]: 131% [96%, 172%]) and controls (median [IQR]: 100% [90%, 102%]) increased, but was no longer significant. Equal protein loading was verified with an antibody against β-actin. Expression ratios of oxidized proteins vs. β-actin were calculated. The median, the interquartile range (IQR), the minimum and the maximum value of the investigated groups of individuals are given. (B) OxyBlot of pooled protein samples before and after paraquat treatment showing a similar trend as identified by individual measurements.
Figure 3. Morphology of the mitochondrial network.
(A) Images of the mitochondrial network in control and patient fibroblasts demonstrating similar degrees of branching under basal culturing conditions. (B) After treatment with paraquat, the network was less branched in patients and controls. (C) The degree of mitochondrial branching (form factor) was comparable in patients (median [IQR]: 78% [66%, 90%]) and controls (median [IQR]: 100% [73%, 105%]) under standard cell culturing conditions. When treated with paraquat (shaded boxes), the form factor decreased significantly in the mutant samples (median [IQR]: 46% [43%, 54%]). By contrast, a drop seen in controls (median [IQR]: 70% [32%, 84%]) was not significant. (D) Citrate synthase activity in cell lysates. Parkin mutants (median [IQR]: 183% [125%, 232%]) showed significantly higher citrate synthase activities than controls (median [IQR]: 100% [43%, 101%]), indicative of increased mitochondrial mass per cell in the former. Citrate synthase activity in cell lysates was normalized for protein concentration. The median, the interquartile range (IQR), the minimum and the maximum value of the investigated groups of individuals are shown.
Similar articles
- Effect of endogenous mutant and wild-type PINK1 on Parkin in fibroblasts from Parkinson disease patients.
Rakovic A, Grünewald A, Seibler P, Ramirez A, Kock N, Orolicki S, Lohmann K, Klein C. Rakovic A, et al. Hum Mol Genet. 2010 Aug 15;19(16):3124-37. doi: 10.1093/hmg/ddq215. Epub 2010 May 27. Hum Mol Genet. 2010. PMID: 20508036 - Mitochondrial impairment observed in fibroblasts from South African Parkinson's disease patients with parkin mutations.
van der Merwe C, Loos B, Swart C, Kinnear C, Henning F, van der Merwe L, Pillay K, Muller N, Zaharie D, Engelbrecht L, Carr J, Bardien S. van der Merwe C, et al. Biochem Biophys Res Commun. 2014 May 2;447(2):334-40. doi: 10.1016/j.bbrc.2014.03.151. Epub 2014 Apr 8. Biochem Biophys Res Commun. 2014. PMID: 24721425 - Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts.
Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O. Mortiboys H, et al. Ann Neurol. 2008 Nov;64(5):555-65. doi: 10.1002/ana.21492. Ann Neurol. 2008. PMID: 19067348 Free PMC article. - Parkin and PINK1 functions in oxidative stress and neurodegeneration.
Barodia SK, Creed RB, Goldberg MS. Barodia SK, et al. Brain Res Bull. 2017 Jul;133:51-59. doi: 10.1016/j.brainresbull.2016.12.004. Epub 2016 Dec 23. Brain Res Bull. 2017. PMID: 28017782 Free PMC article. Review. - [Parkin and mitochondria].
Mitsui T, Kuroda Y, Kaji R. Mitsui T, et al. Brain Nerve. 2008 Aug;60(8):923-9. Brain Nerve. 2008. PMID: 18717196 Review. Japanese.
Cited by
- Deciphering glycomics and neuroproteomic alterations in experimental traumatic brain injury: Comparative analysis of aspirin and clopidogrel treatment.
Abou-Abbass H, Bahmad H, Abou-El-Hassan H, Zhu R, Zhou S, Dong X, Hamade E, Mallah K, Zebian A, Ramadan N, Mondello S, Fares J, Comair Y, Atweh S, Darwish H, Zibara K, Mechref Y, Kobeissy F. Abou-Abbass H, et al. Electrophoresis. 2016 Jun;37(11):1562-76. doi: 10.1002/elps.201500583. Epub 2016 Mar 29. Electrophoresis. 2016. PMID: 27249377 Free PMC article. - Gene-environment interactions in Parkinson's disease: specific evidence in humans and mammalian models.
Cannon JR, Greenamyre JT. Cannon JR, et al. Neurobiol Dis. 2013 Sep;57:38-46. doi: 10.1016/j.nbd.2012.06.025. Epub 2012 Jul 7. Neurobiol Dis. 2013. PMID: 22776331 Free PMC article. Review. - Investigation of USP30 inhibition to enhance Parkin-mediated mitophagy: tools and approaches.
Tsefou E, Walker AS, Clark EH, Hicks AR, Luft C, Takeda K, Watanabe T, Ramazio B, Staddon JM, Briston T, Ketteler R. Tsefou E, et al. Biochem J. 2021 Dec 10;478(23):4099-4118. doi: 10.1042/BCJ20210508. Biochem J. 2021. PMID: 34704599 Free PMC article. - Spinocerebellar Ataxia Type 28-Phenotypic and Molecular Characterization of a Family with Heterozygous and Compound-Heterozygous Mutations in AFG3L2.
Tunc S, Dulovic-Mahlow M, Baumann H, Baaske MK, Jahn M, Junker J, Münchau A, Brüggemann N, Lohmann K. Tunc S, et al. Cerebellum. 2019 Aug;18(4):817-822. doi: 10.1007/s12311-019-01036-2. Cerebellum. 2019. PMID: 31111429 - Natural variation in Caenorhabditis briggsae mitochondrial form and function suggests a novel model of organelle dynamics.
Hicks KA, Denver DR, Estes S. Hicks KA, et al. Mitochondrion. 2013 Jan;13(1):44-51. doi: 10.1016/j.mito.2012.12.006. Epub 2012 Dec 23. Mitochondrion. 2013. PMID: 23269324 Free PMC article.
References
- Lucking CB, Durr A, Bonifati V, Vaughan J, De Michele G, et al. Association between early-onset Parkinson's disease and mutations in the parkin gene. N Engl J Med. 2000;342:1560–1567. - PubMed
- Moore DJ. Parkin: a multifaceted ubiquitin ligase. Biochem Soc Trans. 2006;34:749–753. - PubMed
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources