Parkin mono-ubiquitinates Bcl-2 and regulates autophagy - PubMed (original) (raw)
Parkin mono-ubiquitinates Bcl-2 and regulates autophagy
Dong Chen et al. J Biol Chem. 2010.
Abstract
Parkin is an E3 ubiquitin ligase that mediates the ubiquitination of protein substrates. The mutations in the parkin gene can lead to a loss of function of parkin and cause autosomal recessive juvenile onset parkinsonism. Recently, parkin was reported to be involved in the regulation of mitophagy. Here, we identify the Bcl-2, an anti-apoptotic and autophagy inhibitory protein, as a substrate for parkin. Parkin directly binds to Bcl-2 via its C terminus and mediates the mono-ubiquitination of Bcl-2, which increases the steady-state levels of Bcl-2. Overexpression of parkin, but not its ligase-deficient forms, decreases autophagy marker LC3 conversion, whereas knockdown of parkin increases LC3 II levels. In HeLa cells, a parkin-deficient cell line, knockdown of parkin does not change LC3 conversion. Moreover, overexpression of parkin enhances the interactions between Bcl-2 and Beclin 1. Our results provide evidence that parkin mono-ubiquitinates Bcl-2 and regulates autophagy via Bcl-2.
Figures
FIGURE 1.
Interactions of parkin and Bcl-2. A, GST-parkin interacts with Bcl-2 in vitro. The supernatant of E. coli crude extract containing recombinant Bcl-2 expressed by pET-21a-Bcl-2 was incubated with glutathione-agarose beads bound with GST or GST-parkin. After incubation, the beads were washed with HNTG buffer, and the bound proteins were probed with anti-Bcl-2 antibody. The lower panels show the inputs of GST, GST-parkin. B, GST-parkin interacts with Bcl-xl in vitro. Similar experiments as in A were performed using GST-parkin to pull down Bcl-xl, which is expressed by pET-21a-Bcl-xl. The lower panels show the inputs of GST, GST-parkin. C, FLAG-parkin is co-immunoprecipitated with EGFP-Bcl-2. 293 cells expressing a combination of EGFP-Bcl-2 and FLAG-parkin or FLAG were subjected to immunoprecipitation with anti-GFP antibody. Immunoprecipitants and inputs were detected with antibodies as indicated. GAPDH served as a loading control. D, endogenous parkin is co-immunoprecipitated with Bcl-2. Mouse brain lysates were subjected to immunoprecipitation with polyclonal antisera against parkin or empty rabbit sera. Immunoprecipitants and inputs were detected with antibodies to parkin and Bcl-2. The parkin and heavy chain bands are overlapped. ▴ indicates the nonspecific bands. E, similar experiments as in A were performed using GST-tagged parkin or its deletion mutants to pull down Bcl-2. Meanwhile, 293 cells expressing a combination of FLAG-Bcl-2 and EGFP-parkin or its deletion mutants were subjected to immunoprecipitation with anti-GFP antibody to further identify the binding domain of parkin with Bcl-2. IP, immunoprecipitation; IB, immunoblot.
FIGURE 2.
Increase of Bcl-2 steady-state levels by parkin mono-ubiquitinating Bcl-2. A, increase of endogenous Bcl-2 by parkin. 293 cells were transfected with EGFP or EGFP-parkin. Cell lysates were subjected to immunoblot analysis using antibodies as indicated. The right panel shows the band intensity of Bcl-2 relative to that of GAPDH. The values are the means ± S.E. from three independent experiments. **, p < 0.01, one-way ANOVA. B, mono-ubiquitination of Bcl-2 by parkin. 293 cells expressing a combination of EGFP-Bcl-2, HA-ubiquitin, FLAG, or FLAG-parkin were subjected to immunoprecipitation using anti-GFP antibody. Immunoprecipitants and inputs were detected with antibodies as indicated. C, parkin increases the stability of Bcl-2. Forty-eight hours after transfection, 293 cells expressing EGFP-Bcl-2 with or without FLAG-parkin were treated with CHX (100 μg/ml). The cells were then harvested at 0, 4, 8, 12, and 16 h and subjected to immunoblot analysis with antibodies to GFP or FLAG. The band density of EGFP-Bcl-2 relative to that of GAPDH was shown. The values are the means ± S.E. from three independent experiments. IP, immunoprecipitation.
FIGURE 3.
Inhibition of autophagy by parkin under normal conditions or starvation. A, 293 cells transfected with EGFP or EGFP-parkin were incubated under normal conditions (control) or starvation (HBSS for 1 h). The cell lysates were subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II, Bcl-2, and Beclin 1 relative to that of GAPDH are shown. The values are the means ± S.E. from three independent experiments. **, p < 0.01; Δ, _p_ > 0.05, one-way ANOVA. B, Parkin inhibits autophagic degradation of long-lived proteins. HeLa cells expressing EGFP or EGFP-parkin were incubated for 24 h with 3H-labeled leucine (1 μCi/ml). The degradation of long-lived proteins was measured at the indicated time points in complete medium or Essential Medium with Earle's Balanced Salts. The values are the means ± S.E. from three independent experiments. con, control.
FIGURE 4.
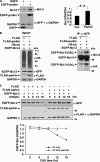
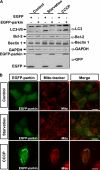
Enhancement of autophagy by parkin upon its mitochondrial localization. A, 293 cells transfected with EGFP or EGFP-parkin were incubated under various conditions, including complete medium (Control), HBSS for 1 h (Starvation), or 10 μ
m
CCCP for 24 h (CCCP). The cell lysates were subjected to immunoblot analysis with antibodies as indicated. B, HeLa cells expressing EGFP-Parkin (green) were treated with Me2SO or HBSS for 1 h, or 10 μ
m
CCCP for 24 h. The cells were then stained with 20 n
m
MitoTracker Red for 15 min and observed using an invert fluorescent microscope.
FIGURE 5.
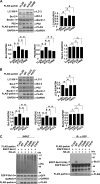
E3 ligase activity-dependent autophagy activation by parkin. A, E3 ligase activity deficient K161N mutant fails to regulate autophagy under normal conditions and starvation treatment (HBSS for 1 h). 293 cells expressing FLAG, FLAG-parkin, or FLAG-parkin (K161N) were subjected to immunoblot analysis with antibodies as indicated. B, Parkin, but not K161N mutant, mono-ubiquitinates Bcl-2 and increases its interactions with Beclin 1. 293 cells expressing a combination of EGFP-Bcl-2, HA-ubiquitin, and FLAG or FLAG-parkin (WT or K161N mutant) were subjected to immunoprecipitation using anti-GFP antibody. Immunoprecipitants and inputs were detected with antibodies as indicated. IP, immunoprecipitation.
FIGURE 6.
Failure to mono-ubiquitinating Bcl-2 and regulating autophagy by disease-linked RING1 and RING2 domain mutants. A and B, disease-linked RING1 and RING2 domain mutants (K161N, T240R, C431F, and P437L) fail to repress autophagy. HeLa cells expressing FLAG, FLAG-parkin, or disease-linked parkin mutants were subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II, Bcl-2, p62, or Beclin 1 relative to that of GAPDH were shown. The values are the means ± S.E. from three independent experiments. **, p < 0.01; *, _p_ < 0.05; Δ, _p_ > 0.05, one-way ANOVA. C, disease-linked parkin mutants fail to mono-ubiquitinate Bcl-2. 293 cells expressing a combination of EGFP-Bcl-2 and HA-ubiquitin together with FLAG-parkin or its RING mutants (K161N, T240R, C431F, and P437L) were subjected to immunoprecipitation using anti-GFP antibody. Immunoprecipitants and inputs were detected with antibodies as indicated. IP, immunoprecipitation.
FIGURE 7.
Increase of autophagy level by knockdown of parkin. A, RNA oligonucleotides against parkin (parkin siRNA) repress parkin expression effectively. The band density of EGFP-Bcl-2 relative to that of GAPDH was shown. The values are the means ± S.E. from three independent experiments. **, p < 0.01, one-way ANOVA. _B_ and _C_, knockdown of parkin enhances autophagy. 293 or SH-SY5Y cells were transfected with siRNAs against parkin or a combination of siRNAs against parkin and EGFP-Bcl-2. Negative control was also set by transfection of control siRNAs. Seventy-two hours after transfection, the total cell lysates were subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II, Bcl-2, and Beclin 1 relative to that of GAPDH were shown. The values are the means ± S.E. from three independent experiments. **, _p_ < 0.01; *, _p_ < 0.05; Δ, _p_ > 0.05, one-way ANOVA. D, knockdown of parkin enhances autophagy in primary cultured neuronal cells from rat HF. Cultured neuronal cells were transfected with siRNAs against parkin or negative control. Seventy-two hours after transfection, the total cell lysates were subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II, Bcl-2, and Beclin 1 relative to that of GAPDH were shown. The values are the means ± S.E. from three independent experiments. **, p < 0.01; *, _p_ < 0.05; Δ, _p_ > 0.05, one-way ANOVA. E, HeLa cells were transfected with siRNAs against parkin or control siRNAs. The total cell lysates were collected 72 h after transfection and then subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II or Beclin 1 relative to that of GAPDH were shown. The values are the means ± S.E. from three independent experiments. Δ, p > 0.05, one-way ANOVA. F, knockdown of Bcl-2 attenuates parkin-induced down-regulation of autophagy. 293 cells were transfected with the RNA oligonucleotides against Bcl-2 (Bcl-2 siRNA) or control siRNA. The cells were then transfected with FLAG or FLAG-parkin. The total cell lysates were collected 72 h after transfection and then subjected to immunoblot analysis with antibodies as indicated. The band densities of LC3-II, Bcl-2, and Beclin 1 relative to that of GAPDH were shown. The values are the means ± S.E. from three independent experiments. **, p < 0.01; *, _p_ < 0.05; Δ, _p_ > 0.05, one-way ANOVA. con, control.
Similar articles
- Autophagy inhibitor LRPPRC suppresses mitophagy through interaction with mitophagy initiator Parkin.
Zou J, Yue F, Li W, Song K, Jiang X, Yi J, Liu L. Zou J, et al. PLoS One. 2014 Apr 10;9(4):e94903. doi: 10.1371/journal.pone.0094903. eCollection 2014. PLoS One. 2014. PMID: 24722279 Free PMC article. - Ubiquitin-specific protease USP36 knockdown impairs Parkin-dependent mitophagy via downregulation of Beclin-1-associated autophagy-related ATG14L.
Geisler S, Jäger L, Golombek S, Nakanishi E, Hans F, Casadei N, Terradas AL, Linnemann C, Kahle PJ. Geisler S, et al. Exp Cell Res. 2019 Nov 15;384(2):111641. doi: 10.1016/j.yexcr.2019.111641. Epub 2019 Sep 21. Exp Cell Res. 2019. PMID: 31550441 - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - Mitochondrion-associated protein LRPPRC suppresses the initiation of basal levels of autophagy via enhancing Bcl-2 stability.
Zou J, Yue F, Jiang X, Li W, Yi J, Liu L. Zou J, et al. Biochem J. 2013 Sep 15;454(3):447-57. doi: 10.1042/BJ20130306. Biochem J. 2013. PMID: 23822101 Free PMC article. - Bcl-2 inhibition of autophagy: a new route to cancer?
Pattingre S, Levine B. Pattingre S, et al. Cancer Res. 2006 Mar 15;66(6):2885-8. doi: 10.1158/0008-5472.CAN-05-4412. Cancer Res. 2006. PMID: 16540632 Review.
Cited by
- Functional implications of mitochondrial reactive oxygen species generated by oncogenic viruses.
Choi YB, Harhaj EW. Choi YB, et al. Front Biol (Beijing). 2014 Dec;9(6):423-436. doi: 10.1007/s11515-014-1332-0. Front Biol (Beijing). 2014. PMID: 25580106 Free PMC article. - Alterations in the E3 ligases Parkin and CHIP result in unique metabolic signaling defects and mitochondrial quality control issues.
Lizama BN, Palubinsky AM, McLaughlin B. Lizama BN, et al. Neurochem Int. 2018 Jul;117:139-155. doi: 10.1016/j.neuint.2017.08.013. Epub 2017 Aug 26. Neurochem Int. 2018. PMID: 28851515 Free PMC article. Review. - Mitochondrial quality control mediated by PINK1 and Parkin: links to parkinsonism.
Narendra D, Walker JE, Youle R. Narendra D, et al. Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a011338. doi: 10.1101/cshperspect.a011338. Cold Spring Harb Perspect Biol. 2012. PMID: 23125018 Free PMC article. - The AMPK-Parkin axis negatively regulates necroptosis and tumorigenesis by inhibiting the necrosome.
Lee SB, Kim JJ, Han SA, Fan Y, Guo LS, Aziz K, Nowsheen S, Kim SS, Park SY, Luo Q, Chung JO, Choi SI, Aziz A, Yin P, Tong SY, Fiesel FC, Springer W, Zhang JS, Lou Z. Lee SB, et al. Nat Cell Biol. 2019 Aug;21(8):940-951. doi: 10.1038/s41556-019-0356-8. Epub 2019 Jul 29. Nat Cell Biol. 2019. PMID: 31358971 Free PMC article. - RBR E3 ubiquitin ligases: new structures, new insights, new questions.
Spratt DE, Walden H, Shaw GS. Spratt DE, et al. Biochem J. 2014 Mar 15;458(3):421-37. doi: 10.1042/BJ20140006. Biochem J. 2014. PMID: 24576094 Free PMC article. Review.
References
- Huang Y., Cheung L., Rowe D., Halliday G. (2004) Brain Res. Brain Res. Rev. 46, 44–70 - PubMed
- Olanow C. W., Tatton W. G. (1999) Annu. Rev. Neurosci. 22, 123–144 - PubMed
- West A. B., Maidment N. T. (2004) Hum. Genet. 114, 327–336 - PubMed
- Lücking C. B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B. S., Meco G., Denèfle P., Wood N. W., Agid Y., Brice A. (2000) N. Engl. J. Med. 342, 1560–1567 - PubMed
- Scott W. K., Nance M. A., Watts R. L., Hubble J. P., Koller W. C., Lyons K., Pahwa R., Stern M. B., Colcher A., Hiner B. C., Jankovic J., Ondo W. G., Allen F. H., Jr., Goetz C. G., Small G. W., Masterman D., Mastaglia F., Laing N. G., Stajich J. M., Slotterbeck B., Booze M. W., Ribble R. C., Rampersaud E., West S. G., Gibson R. A., Middleton L. T., Roses A. D., Haines J. L., Scott B. L., Vance J. M., Pericak-Vance M. A. (2001) J. Am. Med. Assoc. 286, 2239–2244 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases