Plasticity of brain networks in a randomized intervention trial of exercise training in older adults - PubMed (original) (raw)
doi: 10.3389/fnagi.2010.00032. eCollection 2010.
Ruchika S Prakash, Kirk I Erickson, Chandramallika Basak, Laura Chaddock, Jennifer S Kim, Heloisa Alves, Susie Heo, Amanda N Szabo, Siobhan M White, Thomas R Wójcicki, Emily L Mailey, Neha Gothe, Erin A Olson, Edward McAuley, Arthur F Kramer
Affiliations
- PMID: 20890449
- PMCID: PMC2947936
- DOI: 10.3389/fnagi.2010.00032
Plasticity of brain networks in a randomized intervention trial of exercise training in older adults
Michelle W Voss et al. Front Aging Neurosci. 2010.
Abstract
Research has shown the human brain is organized into separable functional networks during rest and varied states of cognition, and that aging is associated with specific network dysfunctions. The present study used functional magnetic resonance imaging (fMRI) to examine low-frequency (0.008 < f < 0.08 Hz) coherence of cognitively relevant and sensory brain networks in older adults who participated in a 1-year intervention trial, comparing the effects of aerobic and non-aerobic fitness training on brain function and cognition. Results showed that aerobic training improved the aging brain's resting functional efficiency in higher-level cognitive networks. One year of walking increased functional connectivity between aspects of the frontal, posterior, and temporal cortices within the Default Mode Network and a Frontal Executive Network, two brain networks central to brain dysfunction in aging. Length of training was also an important factor. Effects in favor of the walking group were observed only after 12 months of training, compared to non-significant trends after 6 months. A non-aerobic stretching and toning group also showed increased functional connectivity in the DMN after 6 months and in a Frontal Parietal Network after 12 months, possibly reflecting experience-dependent plasticity. Finally, we found that changes in functional connectivity were behaviorally relevant. Increased functional connectivity was associated with greater improvement in executive function. Therefore the study provides the first evidence for exercise-induced functional plasticity in large-scale brain systems in the aging brain, using functional connectivity techniques, and offers new insight into the role of aerobic fitness in attenuating age-related brain dysfunction.
Keywords: aerobic fitness; aging; default mode network; executive function; exercise; fMRI; functional connectivity.
Figures
Figure 1
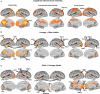
Mean statistical maps for the average of old and young subjects, for cognitive networks, are illustrated in Figure 1A, followed by statistical maps for the contrasts of Young > Old and Old > Young in Figures 1B and 1C, respectively.
Figure 2
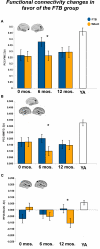
Significant effects in favor of the FTB group, in regions reflecting age-related network disruption, in the DMN. (A, B) and the FP network (C), are visualized by plotting marginal group means from the analysis at the corresponding time point (error bars represent 1 ± standard error of the marginal mean); *p < 0.05. Refer to Table 3 for anatomical description and MNI coordinates of ROIs represented by black circles. For all brains, R = R and L = L.
Figure 3
Significant effects in favor of the walking group, in regions reflecting age-related network disruption, in the DMN. (A–C) and the FE network (D), are visualized by plotting marginal group means from the analysis at the corresponding time point (error bars represent 1 ± standard error of the marginal mean); *p < 0.05. Refer to Table 3 for anatomical description and MNI coordinates of ROIs represented by black circles. For all brains, R = R and L = L.
Figure 4
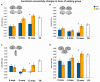
Exercise training-related changes in functional connectivity in regions where old started greater than young. Significant effects, from FE network, visualized by plotting marginal group means from the analysis at the corresponding time point (error bars represent 1 + standard error of the marginal mean); *p < 0.05. Refer to Table 4 for anatomical description and MNI coordinates of ROIs represented by black circles. For all brains, R = R and L = L.
Similar articles
- Cardiorespiratory fitness predicts effective connectivity between the hippocampus and default mode network nodes in young adults.
Kronman CA, Kern KL, Nauer RK, Dunne MF, Storer TW, Schon K. Kronman CA, et al. Hippocampus. 2020 May;30(5):526-541. doi: 10.1002/hipo.23169. Epub 2019 Oct 24. Hippocampus. 2020. PMID: 31647603 Free PMC article. - Effects of a 12-Week Aerobic Spin Intervention on Resting State Networks in Previously Sedentary Older Adults.
McGregor KM, Crosson B, Krishnamurthy LC, Krishnamurthy V, Hortman K, Gopinath K, Mammino KM, Omar J, Nocera JR. McGregor KM, et al. Front Psychol. 2018 Nov 27;9:2376. doi: 10.3389/fpsyg.2018.02376. eCollection 2018. Front Psychol. 2018. PMID: 30542314 Free PMC article. - A Longitudinal Study of Changes in Resting-State Functional Magnetic Resonance Imaging Functional Connectivity Networks During Healthy Aging.
Oschmann M, Gawryluk JR. Oschmann M, et al. Brain Connect. 2020 Sep;10(7):377-384. doi: 10.1089/brain.2019.0724. Epub 2020 Aug 19. Brain Connect. 2020. PMID: 32623915 Free PMC article. - Healthy aging by staying selectively connected: a mini-review.
Antonenko D, Flöel A. Antonenko D, et al. Gerontology. 2014;60(1):3-9. doi: 10.1159/000354376. Epub 2013 Sep 28. Gerontology. 2014. PMID: 24080587 Review. - Three Large-Scale Functional Brain Networks from Resting-State Functional MRI in Subjects with Different Levels of Cognitive Impairment.
Joo SH, Lim HK, Lee CU. Joo SH, et al. Psychiatry Investig. 2016 Jan;13(1):1-7. doi: 10.4306/pi.2016.13.1.1. Epub 2015 Nov 20. Psychiatry Investig. 2016. PMID: 26766941 Free PMC article. Review.
Cited by
- Age-related changes in parietal lobe activation during an episodic memory retrieval task.
Oedekoven CS, Jansen A, Kircher TT, Leube DT. Oedekoven CS, et al. J Neural Transm (Vienna). 2013 May;120(5):799-806. doi: 10.1007/s00702-012-0904-x. Epub 2012 Oct 20. J Neural Transm (Vienna). 2013. PMID: 23086222 - Subjective memory impairment and well-being in community-dwelling older adults.
Zuniga KE, Mackenzie MJ, Kramer A, McAuley E. Zuniga KE, et al. Psychogeriatrics. 2016 Jan;16(1):20-6. doi: 10.1111/psyg.12112. Epub 2015 Mar 3. Psychogeriatrics. 2016. PMID: 25737426 Free PMC article. Clinical Trial. - Exercise Intervention for Alzheimer's Disease: Unraveling Neurobiological Mechanisms and Assessing Effects.
Ren J, Xiao H. Ren J, et al. Life (Basel). 2023 Nov 30;13(12):2285. doi: 10.3390/life13122285. Life (Basel). 2023. PMID: 38137886 Free PMC article. Review. - Dose-Response Association of Tai Chi and Cognition among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis.
Chen ML, Wotiz SB, Banks SM, Connors SA, Shi Y. Chen ML, et al. Int J Environ Res Public Health. 2021 Mar 19;18(6):3179. doi: 10.3390/ijerph18063179. Int J Environ Res Public Health. 2021. PMID: 33808633 Free PMC article. - Physical Activity Is Linked to Greater Moment-To-Moment Variability in Spontaneous Brain Activity in Older Adults.
Burzynska AZ, Wong CN, Voss MW, Cooke GE, Gothe NP, Fanning J, McAuley E, Kramer AF. Burzynska AZ, et al. PLoS One. 2015 Aug 5;10(8):e0134819. doi: 10.1371/journal.pone.0134819. eCollection 2015. PLoS One. 2015. PMID: 26244873 Free PMC article.
References
LinkOut - more resources
Full Text Sources