Aggregation of detergent-insoluble tau is involved in neuronal loss but not in synaptic loss - PubMed (original) (raw)
Aggregation of detergent-insoluble tau is involved in neuronal loss but not in synaptic loss
Tetsuya Kimura et al. J Biol Chem. 2010.
Abstract
Neurofibrillary tangles (NFTs), which consist of highly phosphorylated tau, are hallmarks of neurodegenerative diseases including Alzheimer disease (AD). In neurodegenerative diseases, neuronal dysfunction due to neuronal loss and synaptic loss accompanies NFT formation, suggesting that a process associated with NFT formation may be involved in neuronal dysfunction. To clarify the relationship between the tau aggregation process and synapse and neuronal loss, we compared two lines of mice expressing human tau with or without an aggregation-prone P301L mutation. P301L tau transgenic (Tg) mice exhibited neuronal loss and produced sarcosyl-insoluble tau in old age but did not exhibit synaptic loss and memory impairment. By contrast, wild-type tau Tg mice neither exhibited neuronal loss nor produced sarcosyl-insoluble tau but did exhibit synaptic loss and memory impairment. Moreover, P301L tau was less phosphorylated than wild-type tau, suggesting that the tau phosphorylation state is involved in synaptic loss, whereas the tau aggregation state is involved in neuronal loss. Finally, increasing concentrations of insoluble tau aggregates leads to the formation of fibrillar tau, which causes NFTs to form.
Figures
FIGURE 1.
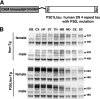
Generation of a transgenic mouse expressing P301L mutant human 2N/4 repeat tau. A, a diagram shows that the expression of mutant P301L human 4 repeat tau tagged with myc and FLAG epitopes was regulated by the CaM kinase II promoter. B, Western blots show the pattern of tau expression in different brain regions. Blots were probed with anti-tauN antibody, which recognizes the N terminus of tau. Male and female mice did not exhibit different expression patterns. OB, olfactory bulb; CE, cerebellum; CX, cerebral cortex; HP, hippocampus; MB, midbrain; SC, spinal cord; ST, striatum; TH, thalamus; MD, medulla.
FIGURE 2.
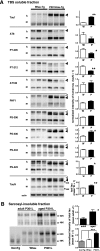
The brains of P301L-Tg mice form sarcosyl-insoluble tau aggregates, but tau from P301L-Tg mice is less phosphorylated than that from Wtau-Tg mice. A, Western blots of TBS-soluble fractions from the brain homogenates of 22-month-old P301Ltau-Tg mice and 22-month-old Wtau-Tg mice (left panel) and histograms show the corresponding tau intensity levels normalized according to TauN immunoreactivity (right panel; W, Wtau-Tg mice; P, P301Ltau-Tg mice). The immunoblots were probed with various anti-tau antibodies, as indicated. Anti-Thr(P)-205, anti-Thr(P)-212, anti-Ser(P)-396, anti-Ser(P)-400, and anti-Ser(P)-422 recognize tau phosphorylated at Thr-205, Thr-212, Ser-396, Ser-400, Ser-404, and Ser-422, respectively. TauN recognizes total phosphorylation-independent tau; AT8 recognizes tau phosphorylated at Ser-199, Ser-202, and Thr-205; AT180 recognizes tau phosphorylated at Thr-231; PHF1 recognizes tau phosphorylated at Ser-396 and Ser-404; actin is shown as an internal control. Open arrowheads indicate the mobility of wild-type human tau bands; filled arrowheads indicate the mobility of the P301L human tau bands. Data are represented as the averages±S.E. *, p < 0.05 (Mann-Whitney test); **, p < 0.01 (Mann-Whitney test). B, upper left panel, Western blots of sarcosyl-insoluble fractions from the brain homogenates of 10-month-old P301Ltau-Tg mice (adult) and 22-month-old P301Ltau-Tg mice (aged) are shown. Immunoreactivities were quantified and represented as averages ± S.E. (n = 5). *, p < 0.05 (Mann-Whitney test) (upper right panel), Lower panel, Western blots are shown of sarcosyl-insoluble fractions from the brain homogenates of aged (22–24 months old) non-Tg, Wtau-Tg, and P301Ltau-Tg mice. Immunoreactivities were quantified and are represented as averages ± S.E. (n = 5). *, p < 0.05; ***, p < 0.005 (Mann-Whitney test) (lower right panel). Immunoreactivities of sarcosyl-insoluble tau in Wtau-Tg mice were similar to levels in non-Tg mice.
FIGURE 3.
The brains of P301Ltau-Tg mice display neuron loss but not NFTs. A, entorhinal cortex sections immunostained with phosphorylation-independent anti-human tau antibody (H-150) or phosphorylation-dependent anti-tau antibodies (AT8 and PHF1) are shown. Phosphorylated human tau accumulated in cell bodies and dendrites of neurons in the entorhinal cortex. B, shown is the entorhinal cortex section stained with the Gallyas silver staining method. No silver-stained neurons were seen in the entorhinal cortex, but silver-stained neurons were occasionally seen in the BLA. C, histograms show the numbers of neurons counted in the TA of neocortex (NC), EC, LA, and BLA of Non-Tg, Wtau-Tg, and P301Ltau-Tg mice. Three cresyl violet-stained brain sections containing each region were analyzed, and the number of neurons in each region was counted using a Neurolucida system (n = 3 mice for each group). For the amygdala, we analyzed sections that were about 50 μm apart, and for neocortex and entorhinal cortex, we analyzed sections that were 300-μm apart. Data are represented as the averages ± S.E. *, p < 0.05 (Mann-Whitney test); **, p < 0.01 (Mann-Whitney test); ***, p < 0.005 (Mann-Whitney test). A and B scale bars, 10 μm.
FIGURE 4.
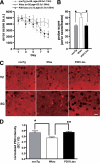
Aged P301Ltau-Tg mice have normal place learning and memory. The Morris water maze was used to assess the place learning and memory of aged (20–24 months old) non-Tg (n = 20), Wtau-Tg (n = 20), and P301Ltau-Tg (n = 13) mice. A, learning and memory performance are expressed as error scores. Learning curves and memory performance of non-Tg and P301Ltau-Tg mice were not significantly different (p < 0.577, _F_ = 0.449, repeated measures two-way analysis of variance). However, Wtau-Tg mice took significantly longer than non-Tg mice to learn the task (_p_ < 0.0326, F = 4.92, repeated measures two-way analysis of variance). _a.u._, arbitrary units. _B_, probe scores of P301Ltau-Tg and non-Tg mice were not different (_p_ > 0.05, Dunn's multiple comparison test). However, the probe scores of Wtau-Tg mice were worse than those of non-Tg mice and P301Ltau-Tg mice (p < 0.05 for each, Dunn's multiple comparison test). Probe score is a measure of memory performance. C, shown are coronal brain sections from non-Tg, Wtau-Tg, and P301Ltau-Tg mice. The sections were immunostained with anti-PSD95 antibody, an antibody against the post-synaptic marker PSD95. Consistent with the learning and memory performance results, PSD95 immunoreactivity in the entorhinal cortex of non-Tg and P301Ltau-Tg mice were not different. However, PSD95 immunoreactivity was reduced in the entorhinal cortex of Wtau-Tg mice. D, shown is quantitative analysis of PSD95 immunoreactivity in entorhinal cortex. Results are expressed as fluorescence intensity of layer I of the lateral entorhinal cortex normalized by that of layer I of ipsilateral visual cortex (non-Tg, n = 7; Wtau-Tg, n = 6; and P301L-Tg, n = 3). Measurements were done on coronal brain sections. Dunn's multiple comparison test revealed a significant difference in PSD95 immunoreactivity between Wtau-Tg and P301L-Tg mice (p < 0.01). Data are represented as averages ± S.E. *, p < 0.05; **, p < 0.01. V2, visual cortex.
Similar articles
- Neurofibrillary tangle formation by introducing wild-type human tau into APP transgenic mice.
Umeda T, Maekawa S, Kimura T, Takashima A, Tomiyama T, Mori H. Umeda T, et al. Acta Neuropathol. 2014 May;127(5):685-98. doi: 10.1007/s00401-014-1259-1. Epub 2014 Feb 15. Acta Neuropathol. 2014. PMID: 24531886 - Changes in dendritic complexity and spine morphology in transgenic mice expressing human wild-type tau.
Dickstein DL, Brautigam H, Stockton SD Jr, Schmeidler J, Hof PR. Dickstein DL, et al. Brain Struct Funct. 2010 Mar;214(2-3):161-79. doi: 10.1007/s00429-010-0245-1. Epub 2010 Mar 7. Brain Struct Funct. 2010. PMID: 20213269 Free PMC article. - Tau protein is cross-linked by transglutaminase in P301L tau transgenic mice.
Halverson RA, Lewis J, Frausto S, Hutton M, Muma NA. Halverson RA, et al. J Neurosci. 2005 Feb 2;25(5):1226-33. doi: 10.1523/JNEUROSCI.3263-04.2005. J Neurosci. 2005. PMID: 15689560 Free PMC article. - [Significance of tau in the development of Alzheimer's disease].
Takashima A. Takashima A. Brain Nerve. 2010 Jul;62(7):701-8. Brain Nerve. 2010. PMID: 20675874 Review. Japanese. - Regulatable transgenic mouse models of Alzheimer disease: onset, reversibility and spreading of Tau pathology.
Hochgräfe K, Sydow A, Mandelkow EM. Hochgräfe K, et al. FEBS J. 2013 Sep;280(18):4371-81. doi: 10.1111/febs.12250. Epub 2013 Apr 22. FEBS J. 2013. PMID: 23517246 Review.
Cited by
- Soluble forms of tau are toxic in Alzheimer's disease.
Kopeikina KJ, Hyman BT, Spires-Jones TL. Kopeikina KJ, et al. Transl Neurosci. 2012 Sep;3(3):223-233. doi: 10.2478/s13380-012-0032-y. Transl Neurosci. 2012. PMID: 23029602 Free PMC article. - Distribution of endogenous normal tau in the mouse brain.
Kubo A, Misonou H, Matsuyama M, Nomori A, Wada-Kakuda S, Takashima A, Kawata M, Murayama S, Ihara Y, Miyasaka T. Kubo A, et al. J Comp Neurol. 2019 Apr 1;527(5):985-998. doi: 10.1002/cne.24577. Epub 2018 Dec 5. J Comp Neurol. 2019. PMID: 30408165 Free PMC article. - Stress acts cumulatively to precipitate Alzheimer's disease-like tau pathology and cognitive deficits.
Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, Sousa N, Almeida OF. Sotiropoulos I, et al. J Neurosci. 2011 May 25;31(21):7840-7. doi: 10.1523/JNEUROSCI.0730-11.2011. J Neurosci. 2011. PMID: 21613497 Free PMC article. - Ectopic Expression Induces Abnormal Somatodendritic Distribution of Tau in the Mouse Brain.
Kubo A, Ueda S, Yamane A, Wada-Kakuda S, Narita M, Matsuyama M, Nomori A, Takashima A, Kato T, Onodera O, Goto M, Ito M, Tomiyama T, Mori H, Murayama S, Ihara Y, Misonou H, Miyasaka T. Kubo A, et al. J Neurosci. 2019 Aug 21;39(34):6781-6797. doi: 10.1523/JNEUROSCI.2845-18.2019. Epub 2019 Jun 24. J Neurosci. 2019. PMID: 31235644 Free PMC article. - Pharmacological Mechanisms Underlying the Neuroprotective Effects of Alpinia oxyphylla Miq. on Alzheimer's Disease.
Xu J, Wang F, Guo J, Xu C, Cao Y, Fang Z, Wang Q. Xu J, et al. Int J Mol Sci. 2020 Mar 18;21(6):2071. doi: 10.3390/ijms21062071. Int J Mol Sci. 2020. PMID: 32197305 Free PMC article.
References
- Gómez-Isla T., Hollister R., West H., Mui S., Growdon J. H., Petersen R. C., Parisi J. E., Hyman B. T. (1997) Ann. Neurol. 41, 17–24 - PubMed
- Ingelsson M., Fukumoto H., Newell K. L., Growdon J. H., Hedley-Whyte E. T., Frosch M. P., Albert M. S., Hyman B. T., Irizarry M. C. (2004) Neurology 62, 925–931 - PubMed
- Goedert M., Spillantini M. G. (2000) Biochim. Biophys. Acta 1502, 110–121 - PubMed
- Hutton M. (2000) Ann. N.Y. Acad. Sci. 920, 63–73 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous