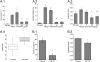Correction of respiratory disorders in a mouse model of Rett syndrome - PubMed (original) (raw)
Correction of respiratory disorders in a mouse model of Rett syndrome
Ana P L Abdala et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Rett syndrome (RTT) is an autism spectrum disorder caused by mutations in the X-linked gene that encodes the transcription factor methyl-CpG-binding protein 2 (MeCP2). A major debilitating phenotype in affected females is frequent apneas, and heterozygous Mecp2-deficient female mice mimic the human respiratory disorder. GABA defects have been demonstrated in the brainstem of Mecp2-deficient mice. Here, using an intact respiratory network, we show that apnea in RTT mice is characterized by excessive excitatory activity in expiratory cranial and spinal nerves. Augmenting GABA markedly improves the respiratory phenotype. In addition, a serotonin 1a receptor agonist that depresses expiratory neuron activity also reduces apnea, corrects the irregular breathing pattern, and prolongs survival in MeCP2 null males. Combining a GABA reuptake blocker with a serotonin 1a agonist in heterozygous females completely corrects their respiratory defects. The results indicate that GABA and serotonin 1a receptor activity are candidates for treatment of the respiratory disorders in Rett syndrome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
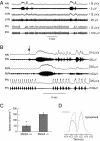
Fig. 1.
Characterization of apnea in Mecp2−/+ mice studied in situ. (A) Representative traces show increased post-I activity in cVN that terminates before end of PN apnea and marked increase in HN that persists throughout PN apnea. (B) Example of tonic HN accompanied by tonic AbN during PN apnea. Note expiratory activity in one HN burst before the apnea (arrowed) and in several after PN apnea. (C) Incidence of apneas in Mecp2+/+ (n = 16) and Mecp2−/+ (n = 17) mice studied in situ. *P ≤ 0.0001 (unpaired t test). (D) Cycle triggered average of 20 consecutive cycles for integrated HN activity superimposed on integrated PN activity recorded in situ. There is significant HN activity during postinspiration.
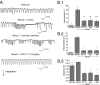
Fig. 2.
Effect of augmenting GABA on respiratory disorders in conscious, freely moving Mecp2−/+ mice. (A) Representative pleural pressure records by telemetry for WT and Mecp2−/+ showing isolated apnea (breath interval ≥ 1.0 s), periodic breathing, and effect of the GABA transporter blocker NO-711; inspiration is downward. (B) Effect of NO-711, diazepam (Diaz, a broad-spectrum GABAA receptor allosteric modulator), and L-838,417 (L-838, a derivative that is relatively specific for receptors that contain α2, α3, and α5 receptor subunits). (B.1) Apnea. WT. *P = 0.002 vs. Mecp2−/+ vehicle; **P = 0.008 vs., Mecp2−/+ vehicle; ***P = 0.015 vs. Mecp2−/+ vehicle; ****P = 0.028 vs. Mecp2−/+ vehicle (n = 9 for WT, n = 8 for vehicle, n = 5 for treatments) (two-way repeated-measures ANOVA with strain and treatment as the two factors). (B.2) Periodic breathing. *P = <0.001 vs. Mecp2−/+ vehicle. (B.3) Irregularity score. *P = <0.001 vs. Mecp2−/+ vehicle; **P = 0.022 vs. Mecp2−/+ vehicle.
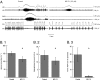
Fig. 4.
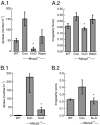
Effect of blocking GABA reuptake and of the serotonin 1a receptor agonist 8-OH-DPAT on respiratory disorders in Mecp2 null male mice. (A) Dose-dependent effect of 8-OH-DPAT on apnea, periodic breathing, and irregularity score. (A.1) Apnea. *P = <0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.046 and 0.02 vs. Mecp2-/y 0 mg/kg (n = 9 for WT, 10 for Mecp2-/y 0 and 300 μg/kg, and 4 for Mecp2-/y 75 and 150 μg/kg) (two-way repeated-measures ANOVA with strain and treatment as the two factors). (A.2) Periodic breathing. *P ≤ 0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.003 and <0.001 vs. Mecp2-/y 0 mg/kg. (A.3) Irregularity score. *P = 0.001 vs. Mecp2-/y 0 mg/kg; **P = 0.048 vs. Mecp2-/y 0 mg/kg; ***P = 0.009 vs. Mecp2-/y 0 mg/kg. (A.4) Box plot for untreated (n = 22) and Mecp2 null males treated with 8-OH-DPAT osmotic pump. P = 0.035 (n = 9) (Kaplan–Meier log–rank test). (B) Effect of NO-711 (5 mg/kg i.p.) on apnea and irregularity score. (B.1) Apnea. *P = 0.001 (n = 4) (paired t test). (B.2) Irregularity score. *P = 0.004.
Fig. 3.
Effect of NO-711 on residual apneas in Mecp2−/+ mice studied in situ. (A) HN, AbN, and PN traces before (control) and after adding NO-711 (5.2 μM) to the perfusate. (B.1–B.3) Effect of the GABA reuptake blocker on (B.1) the incidence of apnea (*P = 0.009, n = 5, paired t test), (B.2) the HN burst envelope in residual PN apneas (*P = 0.005, n = 5), and (B.3) the incidence of apneas that contained tonic AbN activity (*P = 0.04, n = 4).
Fig. 5.
Effect of combined GABA reuptake block and serotonin 1a agonist on apnea and irregularity score in Mecp2−/+ studied in situ and conscious Mecp2-/y mice. (A.1) Apnea in Mecp2−/+ female mice treated with combined NO-711 (5.2 μM) and 8-OH-DPAT (0.1 μM). *P = 0.01 (n = 6) (ANOVA). (A.2) Irregularity score. *P = 0.001. (B.1) Apnea in Mecp2-/y males treated with combined NO-711 (1.0 mg/kg) and 8-OH-DPAT (150 μg/kg). *P = 0.016 (n = 5) (paired t test). (B.2) Irregularity score. *P = 0.05.
Similar articles
- Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome.
Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JF. Abdala AP, et al. J Physiol. 2016 Jan 1;594(1):223-37. doi: 10.1113/JP270966. Epub 2015 Dec 14. J Physiol. 2016. PMID: 26507912 Free PMC article. - A BDNF loop-domain mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome.
Kron M, Lang M, Adams IT, Sceniak M, Longo F, Katz DM. Kron M, et al. Dis Model Mech. 2014 Sep;7(9):1047-55. doi: 10.1242/dmm.016030. Dis Model Mech. 2014. PMID: 25147297 Free PMC article. - Defective GABAergic neurotransmission in the nucleus tractus solitarius in Mecp2-null mice, a model of Rett syndrome.
Chen CY, Di Lucente J, Lin YC, Lien CC, Rogawski MA, Maezawa I, Jin LW. Chen CY, et al. Neurobiol Dis. 2018 Jan;109(Pt A):25-32. doi: 10.1016/j.nbd.2017.09.006. Epub 2017 Sep 18. Neurobiol Dis. 2018. PMID: 28927958 Free PMC article. - Exploring the possible link between MeCP2 and oxidative stress in Rett syndrome.
Filosa S, Pecorelli A, D'Esposito M, Valacchi G, Hajek J. Filosa S, et al. Free Radic Biol Med. 2015 Nov;88(Pt A):81-90. doi: 10.1016/j.freeradbiomed.2015.04.019. Epub 2015 May 8. Free Radic Biol Med. 2015. PMID: 25960047 Review. - Sex differences in Mecp2-mutant Rett syndrome model mice and the impact of cellular mosaicism in phenotype development.
Ribeiro MC, MacDonald JL. Ribeiro MC, et al. Brain Res. 2020 Feb 15;1729:146644. doi: 10.1016/j.brainres.2019.146644. Epub 2020 Jan 2. Brain Res. 2020. PMID: 31904347 Free PMC article. Review.
Cited by
- Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome.
Ren J, Ding X, Funk GD, Greer JJ. Ren J, et al. J Neurosci. 2012 Nov 28;32(48):17230-40. doi: 10.1523/JNEUROSCI.2951-12.2012. J Neurosci. 2012. PMID: 23197715 Free PMC article. - Loss of MeCP2 in the rat models regression, impaired sociability and transcriptional deficits of Rett syndrome.
Veeraragavan S, Wan YW, Connolly DR, Hamilton SM, Ward CS, Soriano S, Pitcher MR, McGraw CM, Huang SG, Green JR, Yuva LA, Liang AJ, Neul JL, Yasui DH, LaSalle JM, Liu Z, Paylor R, Samaco RC. Veeraragavan S, et al. Hum Mol Genet. 2016 Aug 1;25(15):3284-3302. doi: 10.1093/hmg/ddw178. Epub 2016 Jun 30. Hum Mol Genet. 2016. PMID: 27365498 Free PMC article. - Cardiorespiratory coupling in health and disease.
Garcia AJ 3rd, Koschnitzky JE, Dashevskiy T, Ramirez JM. Garcia AJ 3rd, et al. Auton Neurosci. 2013 Apr;175(1-2):26-37. doi: 10.1016/j.autneu.2013.02.006. Epub 2013 Mar 13. Auton Neurosci. 2013. PMID: 23497744 Free PMC article. Review. - Effect of Sarizotan, a 5-HT1a and D2-like receptor agonist, on respiration in three mouse models of Rett syndrome.
Abdala AP, Lioy DT, Garg SK, Knopp SJ, Paton JF, Bissonnette JM. Abdala AP, et al. Am J Respir Cell Mol Biol. 2014 Jun;50(6):1031-9. doi: 10.1165/rcmb.2013-0372OC. Am J Respir Cell Mol Biol. 2014. PMID: 24351104 Free PMC article. - Rett syndrome: insights into genetic, molecular and circuit mechanisms.
Ip JPK, Mellios N, Sur M. Ip JPK, et al. Nat Rev Neurosci. 2018 Jun;19(6):368-382. doi: 10.1038/s41583-018-0006-3. Nat Rev Neurosci. 2018. PMID: 29740174 Free PMC article. Review.
References
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Bird A. The methyl-CpG-binding protein MeCP2 and neurological disease. Biochem Soc Trans. 2008;36:575–583. - PubMed
- Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases