Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension - PubMed (original) (raw)
Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension
Juliette Hadchouel et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Mutations in WNK1 and WNK4 lead to familial hyperkalemic hypertension (FHHt). Because FHHt associates net positive Na(+) balance together with K(+) and H(+) renal retention, the identification of WNK1 and WNK4 led to a new paradigm to explain how aldosterone can promote either Na(+) reabsorption or K(+) secretion in a hypovolemic or hyperkalemic state, respectively. WNK1 gives rise to L-WNK1, an ubiquitous kinase, and KS-WNK1, a kinase-defective isoform expressed in the distal convoluted tubule. By inactivating KS-WNK1 in mice, we show here that this isoform is an important regulator of sodium transport. KS-WNK1(-/-) mice display an increased activity of the Na-Cl cotransporter NCC, expressed specifically in the distal convoluted tubule, where it participates in the fine tuning of sodium reabsorption. Moreover, the expression of the ROMK and BKCa potassium channels was modified in KS-WNK1(-/-) mice, indicating that KS-WNK1 is also a regulator of potassium transport in the distal nephron. Finally, we provide an alternative model for FHHt. Previous studies suggested that the activation of NCC plays a central role in the development of hypertension and hyperkalemia. Even though the increase in NCC activity in KS-WNK1(-/-) mice was less pronounced than in mice overexpressing a mutant form of WNK4, our study suggests that the activation of Na-Cl cotransporter is not sufficient by itself to induce a hyperkalemic hypertension and that the deregulation of other channels, such as the Epithelial Na(+) channel (ENaC), is probably required.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Increased diastolic blood pressure and mean arterial pressure in KS-WNK1−/− mice. Profiles over 24 h in systolic blood pressure (SBP) and diastolic blood pressure (DBP) under a 12-h/12-h day/night schedule (0800 to 2000 hours and 2000 to 0800 hours) in KS-WNK+/+ (n = 5) and KS-WNK−/− (n = 5) mice instrumented with a telemetric system under basal condition. Data are means ± SEM.
Fig. 2.
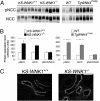
Expression and phosphorylation of NCC is increased in KS-WNK1−/− mice but to a lesser extent than in TgWNK4PHAII mice. (A) Immunoblots of samples dissected from the renal cortex of control and KS-WNK1−/− males (n = 6 per group; Left) or control and TgWNK4PHAII males (n = 4 and n = 5, respectively; Right) incubated with anti-NCC and anti-pNCC antibodies. (B) Densitometric analysis showed that abundance and phosphorylation of NCC were significantly increased in KS-WNK1−/− mice (black bars; *P < 0.05) compared with controls (open bars), but this activation was lower than that seen in TgWNK4PHAII mice (gray bars; **P < 0.005) compared with their controls (open bars). Data are mean ± SEM. (C) Cryostat sections of KS-WNK1+/+ and KS-WNK1−/− male kidneys immunostained with anti-NCC antibody. The signal appears stronger in the DCT of KS-WNK1−/− mice compared with control littermates. (Scale bar: 50 μm.)
Fig. 3.
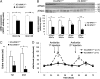
Expression and activity of ENaC in KS-WNK1−/− mice. (A) Quantification of the expression level of Scnn1a and Scnn1b transcripts in the kidney and renal cortex of KS-WNK1−/− mice (n = 10) and control littermates (n = 5) by real-time quantitative RT-PCR showed that expression of these ENaC subunits is significantly decreased in KS-WNK1−/− males (*P < 0.05, **P < 0.005). Results are expressed in arbitrary units relative to the expression of 18S and the expression level in KS-WNK1+/+ mice was arbitrarily set to one. (B) Semiquantitative immunoblotting of membrane fractions from cortex dissected from control and KS-WNK1−/− male kidneys. (Upper) Immunoblots of samples from control and KS-WNK1−/− male mice (n = 7 per group) incubated with anti-α and anti-γ subunits of ENaC antibody. (Lower) Densitometric analysis showed that the abundance of the complete (85 kDa) and cleaved (30 kDa) form of αENaC and the cleaved 70-kDa form but not the complete 85-kDa form of γENaC was significantly decreased in KS-WNK1−/− mice (filled bars; *P < 0.05, **P < 0.005) compared with control mice (open bars). (C) Urinary output in response to amiloride injections. The reported volume corresponds to the first 6-h urine collection after injection (Methods). There was a trend toward decreased diuresis in response to amiloride in KS-WNK1−/− male mice after the first injection, which became significant after the second injection (*P < 0.05). (D) Urinary Na+ excretion in response to amiloride injection. The amiloride-induced natriuresis appeared to be blunted in KS-WNK1−/− male mice compared with KS-WNK1+/+ male mice. Data in all graphs are mean ± SEM.
Fig. 4.
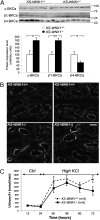
K+ handling in KS-WNK1−/− mice. (A) Semiquantitative immunoblotting of membrane fractions from cortex dissected from control and KS-WNK1−/− male kidneys (n = 7 per group). (Upper) Immunoblots of samples from control and KS-WNK1−/− males incubated with anti-α subunit of the BKCa channel antibody, anti-β1 subunit of the BKCa channel, and anti-β4 subunit of the BKCa channel antibody. (Lower) Densitometric analysis showed that abundance of the α- and β1-subunits of BKCa at the membrane was significantly increased, whereas abundance of the β4-subunit was decreased in KS-WNK1−/− mice (filled bars; *P < 0.05, **P < 0.005) compared with control mice (open bars). (B) ROMK immunostaining in kidneys of two KS-WNK1+/+ and two KS-WNK1−/− mice. The stained tubuli are DCTs and CNTs grouped around the cortical radial vessels. The mutant mice appear to have more apical localization of ROMK than WT mice. However, note the marked variability of apical immunostaining between animals and between tubules within one animal. (Scale bar: 50 μm.) (C) Urinary K+ excretion in response to K+ load. KS-WNK1+/+ and KS-WNK1−/− male mice (n = 6 per group) were housed in metabolic cages and fed a control diet (0.8% K+) for 24 h and then a high-K+ diet (7% KCl; 4% K+) for 10 d. During the normal diet and the first 48 h of K+ load, urine samples were collected every 12 h. There was no difference between KS-WNK1+/+ and KS-WNK1−/− male mice. Data in all graphs are mean ± SEM.
Similar articles
- Downregulation of NCC and NKCC2 cotransporters by kidney-specific WNK1 revealed by gene disruption and transgenic mouse models.
Liu Z, Xie J, Wu T, Truong T, Auchus RJ, Huang CL. Liu Z, et al. Hum Mol Genet. 2011 Mar 1;20(5):855-66. doi: 10.1093/hmg/ddq525. Epub 2010 Dec 2. Hum Mol Genet. 2011. PMID: 21131289 Free PMC article. - Kidney-specific WNK1 isoform (KS-WNK1) is a potent activator of WNK4 and NCC.
Argaiz ER, Chavez-Canales M, Ostrosky-Frid M, Rodríguez-Gama A, Vázquez N, Gonzalez-Rodriguez X, Garcia-Valdes J, Hadchouel J, Ellison D, Gamba G. Argaiz ER, et al. Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F734-F745. doi: 10.1152/ajprenal.00145.2018. Epub 2018 May 30. Am J Physiol Renal Physiol. 2018. PMID: 29846116 Free PMC article. - Deletion of KS-WNK1 promotes NCC activation by increasing WNK1/4 abundance.
Ferdaus MZ, Terker AS, Koumangoye RB, Al-Qusairi L, Welling PA, Delpire E. Ferdaus MZ, et al. Am J Physiol Renal Physiol. 2024 Sep 1;327(3):F373-F385. doi: 10.1152/ajprenal.00101.2024. Epub 2024 Jul 4. Am J Physiol Renal Physiol. 2024. PMID: 38961847 - WNK1 in the kidney.
Bahena-Lopez JP, Gamba G, Castañeda-Bueno M. Bahena-Lopez JP, et al. Curr Opin Nephrol Hypertens. 2022 Sep 1;31(5):471-478. doi: 10.1097/MNH.0000000000000820. Epub 2022 Jul 15. Curr Opin Nephrol Hypertens. 2022. PMID: 35894282 Review. - [New perspective on the role of WNK1 and WNK4 in the regulation of NaCl reabsorption and K(+) secretion by the distal nephron].
Rafael C, Chavez-Canales M, Hadchouel J. Rafael C, et al. Med Sci (Paris). 2016 Mar;32(3):274-80. doi: 10.1051/medsci/20163203012. Epub 2016 Mar 23. Med Sci (Paris). 2016. PMID: 27011246 Review. French.
Cited by
- Dietary potassium and the renal control of salt balance and blood pressure.
Penton D, Czogalla J, Loffing J. Penton D, et al. Pflugers Arch. 2015 Mar;467(3):513-30. doi: 10.1007/s00424-014-1673-1. Epub 2015 Jan 6. Pflugers Arch. 2015. PMID: 25559844 Review. - WNK bodies cluster WNK4 and SPAK/OSR1 to promote NCC activation in hypokalemia.
Thomson MN, Cuevas CA, Bewarder TM, Dittmayer C, Miller LN, Si J, Cornelius RJ, Su XT, Yang CL, McCormick JA, Hadchouel J, Ellison DH, Bachmann S, Mutig K. Thomson MN, et al. Am J Physiol Renal Physiol. 2020 Jan 1;318(1):F216-F228. doi: 10.1152/ajprenal.00232.2019. Epub 2019 Nov 18. Am J Physiol Renal Physiol. 2020. PMID: 31736353 Free PMC article. - Thirty years of the NaCl cotransporter: from cloning to physiology and structure.
Gamba G. Gamba G. Am J Physiol Renal Physiol. 2023 Oct 1;325(4):F479-F490. doi: 10.1152/ajprenal.00114.2023. Epub 2023 Aug 10. Am J Physiol Renal Physiol. 2023. PMID: 37560773 Free PMC article. Review. - Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved.
Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, Gamba G. Castañeda-Bueno M, et al. Am J Physiol Renal Physiol. 2014 Jun 15;306(12):F1507-19. doi: 10.1152/ajprenal.00255.2013. Epub 2014 Apr 23. Am J Physiol Renal Physiol. 2014. PMID: 24761002 Free PMC article. - L-WNK1 is required for BK channel activation in intercalated cells.
Ray EC, Carrisoza-Gaytan R, Al-Bataineh M, Marciszyn AL, Nkashama LJ, Chen J, Winfrey A, Griffiths S, Lam TR, Flores D, Wu P, Wang W, Huang CL, Subramanya AR, Kleyman TR, Satlin LM. Ray EC, et al. Am J Physiol Renal Physiol. 2021 Aug 1;321(2):F245-F254. doi: 10.1152/ajprenal.00472.2020. Epub 2021 Jul 6. Am J Physiol Renal Physiol. 2021. PMID: 34229479 Free PMC article.
References
- Gordon RD, et al. Gordon's syndrome: a sodium-volume-dependent form of hypertension with a genetic basis. In: Laragh JH, Brenner BM, editors. Hypertension: Pathology, Diagnosis and Management. 2nd ed. New York: Raven Press; 1995. pp. 2111–2113.
- Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. - PubMed
- Xu B, et al. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem. 2000;275:16795–16801. - PubMed
- Kahle KT, et al. A novel protein kinase signaling pathway essential for blood pressure regulation in humans. Trends Endocrinol Metab. 2008;19:91–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases