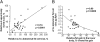Regional differences in cellular mechanisms of adipose tissue gain with overfeeding - PubMed (original) (raw)
Regional differences in cellular mechanisms of adipose tissue gain with overfeeding
Yourka D Tchoukalova et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Body fat distribution is an important predictor of the metabolic consequences of obesity, but the cellular mechanisms regulating regional fat accumulation are unknown. We assessed the changes in adipocyte size (photomicrographs) and number in response to overfeeding in upper- and lower-body s.c. fat depots of 28 healthy, normal weight adults (15 men) age 29 ± 2 y. We analyzed how these changes relate to regional fat gain (dual energy X-ray absorptiometry and computed tomography) and baseline preadipocyte proliferation, differentiation [peroxisome proliferator-activated receptor-γ2 (PPARγ2) and CCAAT/enhancer binding protein-α (C/EBPα) mRNA]), and apoptotic response to TNF-α. Fat mass increased by 1.9 ± 0.2 kg in the upper body and 1.6 ± 0.1 kg in the lower body. Average abdominal s.c. adipocyte size increased by 0.16 ± 0.06 μg lipid per cell and correlated with relative upper-body fat gain (r = 0.74, P < 0.0001). However, lower-body fat responded to overfeeding by fat-cell hyperplasia, with adipocyte number increasing by 2.6 ± 0.9 × 10(9) cells (P < 0.01). We found no depot-differences in preadipocyte replication or apoptosis that would explain lower-body adipocyte hyperplasia and abdominal s.c. adipocyte hypertrophy. However, baseline PPARγ2 and C/EBPα mRNA were higher in abdominal than femoral s.c. preadipocytes (P < 0.005 and P < 0.03, respectively), consistent with the ability of abdominal s.c. adipocytes to achieve a larger size. Inherent differences in preadipocyte cell dynamics may contribute to the distinct responses of different fat depots to overfeeding, and fat-cell number increases in certain depots in adults after only 8 wk of increased food intake.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
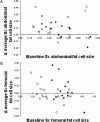
Fig. 1.
Relationship between baseline mature adipocyte cell size (x axis) and change in adipocyte size (y axis) in response to weight gain. •, data from women; □, data from men. (A) Abdominal s.c. adipocytes vs. change in abdominal adipocyte size. The relationship is significant for women (r = −0.64, P = 0.02) but not for men (r = 0.23, P = 0.43). (B) Femoral adipocytes vs. change in femoral adipocyte size. The relationship is significant for women (r = −0.64, P = 0.02) and is of borderline significance for men (r = −0.52, P = 0.057).
Fig. 2.
Relationships between changes in regional s.c. fat gain and changes in adipocyte size. •, data from women; □, data from men. (A) Relative change (%) in upper-body fat mass {[(upper-body fat mass postoverfeeding − upper-body fat mass at baseline) ÷ upper-body fat mass at baseline] × 100} vs. relative change (%) in abdominal s.c. adipocyte size {[(fat-cell size postoverfeeding − fat-cell size at baseline) ÷ fat-cell size at baseline] × 100}. (B) Change in abdominal s.c. fat-cell size vs. the relative amount of lower-body fat gain {[(lower-body fat mass postoverfeeding − lower-body fat mass at baseline) ÷ lower-body fat mass at baseline] × 100}. Because the data were not normally distributed, regression analysis was performed using logarithmically transformed values of the relative increase in lower-body fat gain. The r and P values provided are from linear regression analysis of the transformed data; the depicted regression line is best fit using a power function.
Similar articles
- Sex- and depot-dependent differences in adipogenesis in normal-weight humans.
Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, Kirkland JL, Jensen MD. Tchoukalova YD, et al. Obesity (Silver Spring). 2010 Oct;18(10):1875-80. doi: 10.1038/oby.2010.56. Epub 2010 Mar 18. Obesity (Silver Spring). 2010. PMID: 20300084 Free PMC article. - Changes in adipose tissue physiology during the first two weeks posthatch in chicks from lines selected for low or high body weight.
Xiao Y, Wang G, Gerrard ME, Wieland S, Davis M, Cline MA, Siegel PB, Gilbert ER. Xiao Y, et al. Am J Physiol Regul Integr Comp Physiol. 2019 Jun 1;316(6):R802-R818. doi: 10.1152/ajpregu.00017.2019. Epub 2019 Apr 10. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30969842 Free PMC article. - Deficiency of FcϵR1 Increases Body Weight Gain but Improves Glucose Tolerance in Diet-Induced Obese Mice.
Lee YJ, Liu C, Liao M, Sukhova GK, Shirakawa J, Abdennour M, Iamarene K, Andre S, Inouye K, Clement K, Kulkarni RN, Banks AS, Libby P, Shi GP. Lee YJ, et al. Endocrinology. 2015 Nov;156(11):4047-58. doi: 10.1210/en.2015-1184. Epub 2015 Aug 21. Endocrinology. 2015. PMID: 26295369 Free PMC article. - Adipogenesis and aging: does aging make fat go MAD?
Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Kirkland JL, et al. Exp Gerontol. 2002 Jun;37(6):757-67. doi: 10.1016/s0531-5565(02)00014-1. Exp Gerontol. 2002. PMID: 12175476 Review. - What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications?
Cuthbertson DJ, Steele T, Wilding JP, Halford JC, Harrold JA, Hamer M, Karpe F. Cuthbertson DJ, et al. Int J Obes (Lond). 2017 Jun;41(6):853-865. doi: 10.1038/ijo.2017.4. Epub 2017 Jan 12. Int J Obes (Lond). 2017. PMID: 28077863 Review.
Cited by
- Partial Deficiency of Zfp217 Resists High-Fat Diet-Induced Obesity by Increasing Energy Metabolism in Mice.
Zeng Q, Wang N, Zhang Y, Yang Y, Li S, Zheng R, Chai J, Qiao T, Jiang S. Zeng Q, et al. Int J Mol Sci. 2021 May 20;22(10):5390. doi: 10.3390/ijms22105390. Int J Mol Sci. 2021. PMID: 34065474 Free PMC article. - Insulin action in adipocytes, adipose remodeling, and systemic effects.
Santoro A, McGraw TE, Kahn BB. Santoro A, et al. Cell Metab. 2021 Apr 6;33(4):748-757. doi: 10.1016/j.cmet.2021.03.019. Cell Metab. 2021. PMID: 33826917 Free PMC article. Review. - Short-term regional meal fat storage in nonobese humans is not a predictor of long-term regional fat gain.
Votruba SB, Jensen MD. Votruba SB, et al. Am J Physiol Endocrinol Metab. 2012 May 15;302(9):E1078-83. doi: 10.1152/ajpendo.00414.2011. Epub 2012 Feb 14. Am J Physiol Endocrinol Metab. 2012. PMID: 22338076 Free PMC article. - The Regulation of Adipose Tissue Health by Estrogens.
Steiner BM, Berry DC. Steiner BM, et al. Front Endocrinol (Lausanne). 2022 May 26;13:889923. doi: 10.3389/fendo.2022.889923. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35721736 Free PMC article. Review. - Heterogeneity of white adipose tissue: molecular basis and clinical implications.
Kwok KH, Lam KS, Xu A. Kwok KH, et al. Exp Mol Med. 2016 Mar 11;48(3):e215. doi: 10.1038/emm.2016.5. Exp Mol Med. 2016. PMID: 26964831 Free PMC article. Review.
References
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. - PubMed
- Snijder MB, et al. Independent and opposite associations of waist and hip circumferences with diabetes, hypertension and dyslipidemia: The AusDiab Study. Int J Obes Relat Metab Disord. 2004;28:402–409. - PubMed
- Snijder MB, et al. Hoorn study Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: The Hoorn study. Diabetes Care. 2004;27:372–377. - PubMed
- Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RR00585/RR/NCRR NIH HHS/United States
- M01 RR000585/RR/NCRR NIH HHS/United States
- AG13925/AG/NIA NIH HHS/United States
- P30 DK050456/DK/NIDDK NIH HHS/United States
- DK50456/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 DK045343/DK/NIDDK NIH HHS/United States
- R01 AG013925/AG/NIA NIH HHS/United States
- DK45343/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources