Regulation of Hip1r by epsin controls the temporal and spatial coupling of actin filaments to clathrin-coated pits - PubMed (original) (raw)
. 2010 Nov 1;123(Pt 21):3652-61.
doi: 10.1242/jcs.066852. Epub 2010 Oct 5.
Affiliations
- PMID: 20923836
- PMCID: PMC2964106
- DOI: 10.1242/jcs.066852
Regulation of Hip1r by epsin controls the temporal and spatial coupling of actin filaments to clathrin-coated pits
Rebecca J Brady et al. J Cell Sci. 2010.
Abstract
Recently, it has become clear that the actin cytoskeleton is involved in clathrin-mediated endocytosis. During clathrin-mediated endocytosis, clathrin triskelions and adaptor proteins assemble into lattices, forming clathrin-coated pits. These coated pits invaginate and detach from the membrane, a process that requires dynamic actin polymerization. We found an unexpected role for the clathrin adaptor epsin in regulating actin dynamics during this late stage of coated vesicle formation. In Dictyostelium cells, epsin is required for both the membrane recruitment and phosphorylation of the actin- and clathrin-binding protein Hip1r. Epsin-null and Hip1r-null cells exhibit deficiencies in the timing and organization of actin filaments at clathrin-coated pits. Consequently, clathrin structures persist on the membranes of epsin and Hip1r mutants and the internalization of clathrin structures is delayed. We conclude that epsin works with Hip1r to regulate actin dynamics by controlling the spatial and temporal coupling of actin filaments to clathrin-coated pits. Specific residues in the ENTH domain of epsin that are required for the membrane recruitment and phosphorylation of Hip1r are also required for normal actin and clathrin dynamics at the plasma membrane. We propose that epsin promotes the membrane recruitment and phosphorylation of Hip1r, which in turn regulates actin polymerization at clathrin-coated pits.
Figures
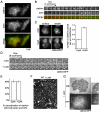
Fig. 1.
Actin polymerization functions in the late stages of clathrin-mediated endocytosis in Dictyostelium. (A) TIRF images of the membrane of wild-type cells expressing clathrinRFP (clathrin) and limEΔcoilGFP (actin). Scale bar: 5 μm. (B) Time-lapse images of the individual clathrinRFP punctum (clathrin) identified in A from appearance to internalization accompanied by a punctum of actin labeled by limEΔcoil (actin). (C) Treatment with the actin depolymerizing drug cytochalasin A leads to an accumulation of clathrin puncta on the plasma membrane. Epifluorescence images from surface and middle focal planes of wild-type cells expressing clathrinRFP. Cells treated with cytochalasin A (**+**cytA) show increased numbers of clathrin puncta on the plasma membrane, 0.89±0.04 puncta per μm, compared to control cells (−cytA), 0.39±0.02 puncta per μm. Mean ± s.e.m., _n_=30 cells. Scale bar: 5 μm. (D) Treatment with the actin depolymerizing drug Latrunculin A treatment causes clathrin pits to persist on the membrane. TIRF time-lapse images of cells expressing clathrinGFP (clathrinGFP) with (+LatA) and without (−LatA) treatment. (E) Quantification of clathrin pits colocalizing with epsin and AP2 simultaneously in the presence (60±2%; _n_=12 cells) and absence (61±4%; _n_=12 cells) of cytochalasin A treatment. (F) Quick-freeze deep etch scanning electron micrograph of assembled clathrin lattices in wild-type cells treated with Latrunculin A (WT + LatA) show that the morphology of clathrin lattices is unaffected. (G) Latrunculin A treatment causes an accumulation of invaginated clathrin-coated pits. Wild-type cells expressing clathrinGFP were treated with Latrunculin A and imaged under IRM and epifluorescence microscopy (GFP). Arrows indicate colocalization between clathrin signal and deeply invaginated pits. All quantification was performed on cells from three independent experiments for each condition.
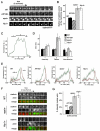
Fig. 2.
Hip1r and epsin, but not AP180, are required for normal clathrin and actin dynamics at the plasma membrane. (A) Clathrin puncta persist at the membrane of epsin and Hip1r-null cells. Time-lapse TIRF images of wild-type (WT), AP180-null (AP180−), epsin-null (epsin−), and Hip1r-null (Hip1r−) cells expressing clathrinRFP. (B) Quantification of the lifetime of clathrin puncta identified at the beginning of TIRF time-lapse acquisition. Wild-type (39±2 seconds, _n_=49; AP180-null (AP180−) 30±2 seconds, _n_=35; epsin-null (epsin−) 70±6 seconds, _n_=31; and Hip1r-null (Hip1r−) 68±4 seconds, _n_=29. (C) Representative plot showing the fluorescence intensity of a wild-type clathrinRFP (clathrin) punctum over time; 1, 2 and 3 mark the assembly, plateau and internalization phases, respectively. (D) Quantification of the average lifetime (seconds) of clathrin puncta from wild-type (_n_=20), AP180-null (_n_=20), epsin-null (_n_=16), and Hip1r-null (_n_=20) cells. Cells are in the assembly phase (1) with normalized intensities of 25–75 a.u.; plateau phase (2) with intensities above 75 a.u.; and internalization phase (3) with intensities of 75–25 a.u. (E) Average plots of the intensity of clathrinRFP (clathrin) puncta over time, with the accompanying actin puncta as labeled by limEΔcoilGFP (actin) in wild-type, AP180-null, epsin-null, and Hip1r-null cells; _n_=16–20 per cell line. (F) Time-lapse TIRF images of individual clathrin and actin puncta in wild-type, AP180-null, epsin-null, and Hip1r-null cells coexpressing clathrinRFP (clathrin) and limEΔcoilGFP (actin). (G) Quantification of laterally mobile actin puncta as labeled by limEΔcoilGFP: wild-type 11±8%, _n_=35 puncta on 5 cells; AP180-null 22±3%, _n_=39 puncta on 6 cells; epsin-null 56±6%, _n_=49 puncta on 5 cells; and Hip1r-null 53±6%, _n_=51 puncta on 6 cells. All values are mean ± s.e.m. All quantification was performed on cells from three independent experiments for each condition.
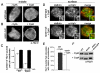
Fig. 3.
Disrupting actin polymerization affects Hip1r localization but not phosphorylation in epsin-null cells. (A) Epifluorescence images from a middle focal plane of wild-type cells (−CytA) and cells treated with cytochalasin A (+CytA), immunostained with anti-Hip1r (α-Hip1r) antibodies. Scale bar: 5 μm. (B) Epifluorescence images from a middle focal plane of epsin-null cells and epsin-null cells treated with cytochalasin A, immunostained with anti-Hip1r antibodies. Note the increased Hip1r localization to the membrane in epsin-null cells treated with cytochalasin A. Scale bar: 5 μm. (C) Quantification of Hip1r localization in wild-type (WT) and epsin-null cells (epsin−) with and without cytochalasin A treatment: WT −cytA, 0.48 ±0.03 puncta per μm; WT +cytA, 0.61±0.02 puncta per μm; epsin− −cytA, 0.16±0.01 puncta per μm; epsin− +cytA, 0.61±0.02 puncta per μm. All values are mean ± s.e.m., _n_=30 cells from three independent experiments for each condition. (D) Epifluorescence images from a surface focal plane of wild-type and epsin-null cells expressing clathrinGFP (clathrin), treated with cytochalasin A, and immunostained for Hip1r. Scale bar: 5 μm. (E) Quantification of colocalization between Hip1r and clathrin in wild-type and epsin-null cells treated with cytochalasin A: wild-type 76±5%, _n_=14 cells; Hip1r-null 67±2%, _n_=20 cells. (F) Western blots of whole cell lysates from wild-type, epsin-null, and epsin-null cells expressing epsinGFP (epsin− + epsinGFP) with or without cytochalasin A treatment. Blots were probed with anti-Hip1r antibodies. Note that cytochalasin A treatment does not induce a phosphorylated species of Hip1r in epsin-null cells.
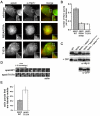
Fig. 4.
EpsinT107A does not rescue the Hip1r and actin-related phenotypes of epsin-null cells. (A) Epifluorescence images from a middle focal plane of epsin-null cells expressing epsinWTGFP (WT), epsinR65A/K78AGFP (R65A/K78A), or epsinT107AGFP (T107A) and immunostained for Hip1r (α-Hip1r). Scale bar: 5 μm. (B) Quantification of Hip1r puncta at the membrane of epsin-null cells expressing epsinWTGFP (epsinWT, 0.40±0.03 puncta per μm), epsinR65A/K78AGFP (epsinR65A/K78A, 0.15±0.01 puncta per μm) epsinT107AGFP (epsinT107A, 13±0.01 puncta per μm); _n_=30 cells from three independent experiments for each cell line. (C) Immunoblots of whole cell lysates from wild-type (WT) and epsin-null (epsin−) cells, and epsin-null cells expressing epsinWTGFP (epsin− + epsinWT), epsinR65A/K78AGFP (epsin− + epsinR65A/K78A), or epsinT107AGFP (epsin− + epsinT107A). Blots were probed with anti-Hip1r (α-Hip1r) or anti-GFP (α-GFP) antibodies. Center row indicates whole-cell lysates treated with calf intestinal phosphatase (CIP). (D) Time-lapse TIRF images of epsin-null cells coexpressing limEΔcoilGFP and either epsinWTGFP or epsinT107AGFP. (E) Quantification of lateral mobility of actin puncta labeled by limEΔcoilGFP in epsin-null cells expressing either epsinWTGFP (21±2%, _n_=23 puncta on three cells) or epsinT107AGFP (43±7%, _n_=30 puncta on three cells).
Similar articles
- Dictyostelium Hip1r contributes to spore shape and requires epsin for phosphorylation and localization.
Repass SL, Brady RJ, O'Halloran TJ. Repass SL, et al. J Cell Sci. 2007 Nov 15;120(Pt 22):3977-88. doi: 10.1242/jcs.011213. Epub 2007 Oct 30. J Cell Sci. 2007. PMID: 17971415 - The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits.
Brady RJ, Wen Y, O'Halloran TJ. Brady RJ, et al. J Cell Sci. 2008 Oct 15;121(Pt 20):3433-44. doi: 10.1242/jcs.032573. Epub 2008 Sep 30. J Cell Sci. 2008. PMID: 18827012 - Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits.
Messa M, Fernández-Busnadiego R, Sun EW, Chen H, Czapla H, Wrasman K, Wu Y, Ko G, Ross T, Wendland B, De Camilli P. Messa M, et al. Elife. 2014 Aug 13;3:e03311. doi: 10.7554/eLife.03311. Elife. 2014. PMID: 25122462 Free PMC article. - Epsin: inducing membrane curvature.
Horvath CA, Vanden Broeck D, Boulet GA, Bogers J, De Wolf MJ. Horvath CA, et al. Int J Biochem Cell Biol. 2007;39(10):1765-70. doi: 10.1016/j.biocel.2006.12.004. Epub 2007 Jan 17. Int J Biochem Cell Biol. 2007. PMID: 17276129 Review. - Myosin VI, a new force in clathrin mediated endocytosis.
Buss F, Luzio JP, Kendrick-Jones J. Buss F, et al. FEBS Lett. 2001 Nov 23;508(3):295-9. doi: 10.1016/s0014-5793(01)03065-4. FEBS Lett. 2001. PMID: 11728438 Review.
Cited by
- Actin and endocytosis in budding yeast.
Goode BL, Eskin JA, Wendland B. Goode BL, et al. Genetics. 2015 Feb;199(2):315-58. doi: 10.1534/genetics.112.145540. Genetics. 2015. PMID: 25657349 Free PMC article. Review. - Genetic association study between STK39 and CCDC62/HIP1R and Parkinson's disease.
Li NN, Tan EK, Chang XL, Mao XY, Zhang JH, Zhao DM, Liao Q, Yu WJ, Peng R. Li NN, et al. PLoS One. 2013 Nov 27;8(11):e79211. doi: 10.1371/journal.pone.0079211. eCollection 2013. PLoS One. 2013. PMID: 24312176 Free PMC article. - Actin polymerization promotes invagination of flat clathrin-coated lattices in mammalian cells by pushing at lattice edges.
Yang C, Colosi P, Hugelier S, Zabezhinsky D, Lakadamyali M, Svitkina T. Yang C, et al. Nat Commun. 2022 Oct 17;13(1):6127. doi: 10.1038/s41467-022-33852-2. Nat Commun. 2022. PMID: 36253374 Free PMC article. - The epsin protein family: coordinators of endocytosis and signaling.
Sen A, Madhivanan K, Mukherjee D, Aguilar RC. Sen A, et al. Biomol Concepts. 2012 Apr;3(2):117-126. doi: 10.1515/bmc-2011-0060. Biomol Concepts. 2012. PMID: 22942912 Free PMC article. - Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi.
Ko G, Paradise S, Chen H, Graham M, Vecchi M, Bianchi F, Cremona O, Di Fiore PP, De Camilli P. Ko G, et al. Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21511-6. doi: 10.1073/pnas.1016390107. Epub 2010 Nov 29. Proc Natl Acad Sci U S A. 2010. PMID: 21115825 Free PMC article.
References
- Aguilar R. C., Watson H. A., Wendland B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737-10743 - PubMed
- Aguilar R. C., Longhi S. A., Shaw J. D., Yeh L. Y., Kim S., Schon A., Freire E., Hsu A., McCormick W. K., Watson H. A., et al. (2006). Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 103, 4116-4121 - PMC - PubMed
- Brady R. J., Wen Y., O'Halloran T. J. (2008). The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits. J. Cell Sci. 121, 3433-3444 - PubMed
- Bretschneider T., Diez S., Anderson K., Heuser J., Clarke M., Muller-Taubenberger A., Kohler J., Gerisch G. (2004). Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14, 1-10 - PubMed
- Chen C. Y., Brodsky F. M. (2005). Huntingtin-interacting protein 1 (Hip1) and Hip1-related protein (Hip1R) bind the conserved sequence of clathrin light chains and thereby influence clathrin assembly in vitro and actin distribution in vivo. J. Biol. Chem. 280, 6109-6117 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases