Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence - PubMed (original) (raw)
Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence
Linda M Wakim et al. Proc Natl Acad Sci U S A. 2010.
Abstract
The brain is not routinely surveyed by lymphocytes and is defined as an immuno-privileged site. However, viral infection of the brain results in the infiltration and long-term persistence of pathogen-specific CD8(+) T cells. These cells survive without replenishment from the circulation and are referred to as resident memory T cells (Trm). Brain Trm selectively express the integrin CD103, the expression of which is dependent on antigen recognition within the tissue. After clearance of virus, CD8(+) T cells persist in tight clusters, presumably at prior infection hot spots. Antigen persistence is not a prerequisite for T-cell retention, as suggested by the failure to detect viral genomes in the T-cell clusters. Furthermore, we show that an intracranial dendritic cell immunization regimen, which allows the transient introduction of antigen, also results in the generation of memory T cells that persist long term in the brain. Brain Trm die rapidly on isolation from the tissue and fail to undergo recall expansion after adoptive transfer into the bloodstream of antigen-challenged recipients. These ex vivo defects imply a dependency on the local milieu for function and survival. Cumulatively, this work shows that Trm are a specialized population of memory T cells that can be deposited in tissues previously thought to be beyond routine immune surveillance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
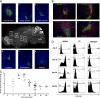
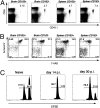
Systemic infection after intranasal VSV infection. Mice were seeded with naïve OT-I.CD45.1 T cells before intranasal (i.n.) infection with VSV-GFP-Ovalbumin. (A) Brains were recovered on day 3 p.i., and virus infected regions were identified based on GFP expression. (B) Brains were recovered on day 8 p.i. and stained with anti-CD45.1 (red), DAPI (blue), and VSV-GFP (green). (C) The graph depicts the number of OT-I cells in the brain at the indicated times postinfection as determined by flow cytometry. Data points represent individual mice, and horizontal bars indicate the mean. (D) The expression of CD103 on the OT-I T cells in the brain, cervical lymph node, and spleen at the indicated days.
Fig. 2.
Memory T cells persisting within the brain after VSV infection are resident. Mice were seeded with naïve OT-I T cells before infection with VSV-OVA (i.n.). On day 20 p.i., CFSE was injected intracranially, and the percentage of CFSE+ OT-I T cells was monitored in the brain, spleen, and deep cervical LN on days (A) 2, (B) 30, and 60 after CFSE injection. Representative flow cytometry profiles are depicted. Values represent the mean ± SEM. (C) Mice were seeded with naïve OT-I T cells before infection with VSV-OVA (i.n.). On day 20 p.i., mice were administered BrdU daily for 1 wk. Shown is the mean amount of BrdU incorporation in OT-I T cells in the spleen, cervical LN, and brain.
Fig. 3.
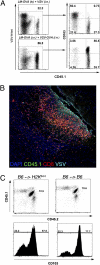
Requirement for antigen recognition within the brain for expression of CD103 on Trm. (A) Mice were seeded with naïve OT-I T cells and were infected with LM-OVA i.v. and either VSV or VSV-OVA i.n. On day 20 p.i., the level of expression of CD103 on OT-I cells (CD45.1) and polyclonal VSV N-specific CD8+ T (VSV-N-tet) cells isolated from the brain was determined. (B) Mice were seeded with naïve OT-I T cells (CD45.1) and were infected with LM-OVA i.v. and VSV i.n. On day 6 p.i., the brain were recovered and stained for VSV antigen (aqua), CD8 (red), CD45.1 (green), and DAPI (blue). (C) Naïve OT-I.CD45.1/2 cells were transferred into either [B6 →H2Kbm1] or [B6 →B6] radiation chimeras before infection with VSV-OVA (i.n.). On day 20 p.i., the level of CD103 expression on OT-I CD8+ T cells in the brain was determined.
Fig. 4.
Retrovirus knockdown of CD103 in OT-I cells reduces Trm accumulation in the brain. RNAi CD103 retrovirus- or control retrovirus-transduced OT-I.CD45.1 transgenic CD8+ T cells (CD45.1+GFP+) were adoptively transferred into naïve mice together with nontransduced OT-I cells (CD45.1+GFP−) followed by VSV-OVA infection. (A) Representative flow cytometry profiles depicting CD103 expression on RNAi CD103-transduced OT-I (GFP+) and nontransduced cells (GFP−) in the brains of mice at the indicated times. (B) The percentage of retrovirus-transduced (either control vector or RNAi CD103) OT-I persisting within the brain after VSV-OVA infection. Data represents the mean ± SEM, with the values indicating the fold reduction.
Fig. 5.
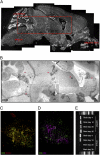
Brain resident memory CD8+ T cells persist in clusters. Brains were recovered from mice on day 21 p.i. with VSV-OVA (i.n.), cut, and stained with (A) DAPI (white), (B) anti-CD8 (black), (C) anti-CD8 (green) and anti-CD103 (red), or (D) anti-CD8 (green) and anti-CD4 (purple). (E) Detection of VSV gRNA in laser-capture microdissected areas of brain on day 4, 10, 20, and 30 p.i. with VSV-OVA.
Fig. 6.
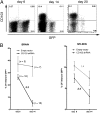
Local DC immunization can generate Trm. OVA peptide-pulsed DCs were injected intracranially into mice seeded with naïve OT-I.CD45.1 CD8+ T cells. (A) The percentage OT-I of the total CD8+ T cell population in the spleen at the indicated times after DC immunization. The bars represent the mean ± SEM. (B) Representative flow cytometry profile depicting the level of expression of CD103 on OT-I cells in the brain on day 30 after DC immunization. (C) The absolute number of CD103+ and CD103− cells in the brain on day 5, 10, and 30 post-DC immunization was determined. The bars represent the mean ± SEM. (D) Immunohistochemistry of the brain staining for CD11c (purple), CD45.1(green), CD103 (red), and DAPI (blue) on day 10 after DC peptide immunization.
Fig. 7.
Resident memory T cells do not function or survive well after dissociation from the tissue in which they reside. Mice were seeded with OT-I.CD45.1 T cells before i.n. infection with VSV-OVA. On day 20 p.i., OT-I cells were recovered from the brain and spleen of mice, sorted into CD103+ and CD103−, and adoptively transferred into naïve recipient mice that were challenged with VSV-OVA (i.n.). (A) Representative flow cytometry profiles of the spleen on day 8 p.i. Numbers represent the proportion of OT-I of the total CD8+ T cell population. (B) Memory OT-I.CD103+ and CD103− cells were sorted from the brain and spleen of mice and cultured in vitro for 12 h. Cells were stained with Annexin V and 7-AAD, and the percentage of dead cells (Annexin V+/− and 7-AAD+) was determined by flow cytometry. (C) A 1:1 ratio of CFSE-labeled peptide-pulsed targets (CFSEhi) and unpulsed (CFSElo) cells were injected intracranially into either naïve mice or mice infected i.n. 14 or 30 d earlier with VSV-OVA. The percentage killing was determined 14 h later.
Similar articles
- Brain-Resident T Cells Following Viral Infection.
Prasad S, Lokensgard JR. Prasad S, et al. Viral Immunol. 2019 Jan/Feb;32(1):48-54. doi: 10.1089/vim.2018.0084. Epub 2018 Sep 18. Viral Immunol. 2019. PMID: 30230418 Free PMC article. Review. - The molecular signature of tissue resident memory CD8 T cells isolated from the brain.
Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, Bevan MJ. Wakim LM, et al. J Immunol. 2012 Oct 1;189(7):3462-71. doi: 10.4049/jimmunol.1201305. Epub 2012 Aug 24. J Immunol. 2012. PMID: 22922816 Free PMC article. - Circulating memory CD8+ T cells are limited in forming CD103+ tissue-resident memory T cells at mucosal sites after reinfection.
Behr FM, Beumer-Chuwonpad A, Kragten NAM, Wesselink TH, Stark R, van Gisbergen KPJM. Behr FM, et al. Eur J Immunol. 2021 Jan;51(1):151-166. doi: 10.1002/eji.202048737. Epub 2020 Aug 31. Eur J Immunol. 2021. PMID: 32762051 - TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection.
Reilly EC, Lambert Emo K, Buckley PM, Reilly NS, Smith I, Chaves FA, Yang H, Oakes PW, Topham DJ. Reilly EC, et al. Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12306-12314. doi: 10.1073/pnas.1915681117. Epub 2020 May 21. Proc Natl Acad Sci U S A. 2020. PMID: 32439709 Free PMC article. - The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.
Corgnac S, Boutet M, Kfoury M, Naltet C, Mami-Chouaib F. Corgnac S, et al. Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
Cited by
- Staphylococcal superantigens evoke temporary and reversible T cell anergy, but fail to block the development of a bacterium specific cellular immune response.
Zhang H, Monk IR, Braverman J, Jones CM, Brooks AG, Stinear TP, Wakim LM. Zhang H, et al. Nat Commun. 2024 Nov 14;15(1):9872. doi: 10.1038/s41467-024-54074-8. Nat Commun. 2024. PMID: 39543088 Free PMC article. - Converging cytokine and metabolite networks shape asymmetric T cell fate at the term human maternal-fetal interface.
Maurice NJ, Erickson JR, DeJong CS, Mair F, Taber AK, Frutoso M, Islas LV, Vigil AB, Lawler RL, McElrath MJ, Newell EW, Sullivan LB, Shree R, McCartney SA. Maurice NJ, et al. bioRxiv [Preprint]. 2024 Jun 12:2024.06.10.598377. doi: 10.1101/2024.06.10.598377. bioRxiv. 2024. PMID: 38915597 Free PMC article. Preprint. - Polyclonal but not monoclonal circulating memory CD4+ T cells attenuate the severity of Staphylococcus aureus bacteremia.
Braverman J, Monk IR, Zhang H, Stinear TP, Wakim LM. Braverman J, et al. Front Immunol. 2024 May 29;15:1417220. doi: 10.3389/fimmu.2024.1417220. eCollection 2024. Front Immunol. 2024. PMID: 38868766 Free PMC article. - Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent Toxoplasma gondii infection.
Porte R, Belloy M, Audibert A, Bassot E, Aïda A, Alis M, Miranda-Capet R, Jourdes A, van Gisbergen KPJM, Masson F, Blanchard N. Porte R, et al. Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2403054121. doi: 10.1073/pnas.2403054121. Epub 2024 Jun 5. Proc Natl Acad Sci U S A. 2024. PMID: 38838017 - Tissue-resident memory T cells: decoding intra-organ diversity with a gut perspective.
Murakami M. Murakami M. Inflamm Regen. 2024 Apr 17;44(1):19. doi: 10.1186/s41232-024-00333-6. Inflamm Regen. 2024. PMID: 38632596 Free PMC article. Review.
References
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. - PubMed
- Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–333. - PubMed
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. - PubMed
- Hou S, Hyland L, Ryan KW, Portner A, Doherty PC. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials