Nuclear speckles - PubMed (original) (raw)
Review
Nuclear speckles
David L Spector et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
Nuclear speckles, also known as interchromatin granule clusters, are nuclear domains enriched in pre-mRNA splicing factors, located in the interchromatin regions of the nucleoplasm of mammalian cells. When observed by immunofluorescence microscopy, they usually appear as 20-50 irregularly shaped structures that vary in size. Speckles are dynamic structures, and their constituents can exchange continuously with the nucleoplasm and other nuclear locations, including active transcription sites. Studies on the composition, structure, and dynamics of speckles have provided an important paradigm for understanding the functional organization of the nucleus and the dynamics of the gene expression machinery.
Figures
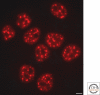
Figure 1.
Speckles form in the interchromatin space. HeLa cells showing splicing factors localized in a speckled pattern as well as being diffusely distributed throughout the nucleoplasm. Bar = 5 µm.
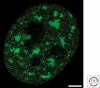
Figure 2.
Structured illumination microscopy, using the OMX system (Applied Precision, Issaqua, Washington), of a HeLa cell expressing SC35-EYFP. At 100 nm resolution substructure can be observed within speckles. In addition, the diffuse population of SC35-EYFP is resolved as a granular distribution. Projection of twelve 0.125 µm optical sections through the center of a nucleus encompassing 1.5 µm. Image provided by Zsolt Lazar and R. Ileng Kumaran. Bar = 2 µm.
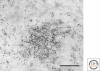
Figure 3.
Nuclear speckles are equivalent to interchromatin granule clusters. Immunoelectron microscopy using a primary antibody against SC35 and a secondary antibody conjugated to 15 nm colloidal gold. IGCs are composed of a series of particles measuring 20–25 nm in diameter that are connected in places by a thin fibril resulting in a beaded chain appearance. Bar = 500 nm.
Similar articles
- Nuclear speckles: a model for nuclear organelles.
Lamond AI, Spector DL. Lamond AI, et al. Nat Rev Mol Cell Biol. 2003 Aug;4(8):605-12. doi: 10.1038/nrm1172. Nat Rev Mol Cell Biol. 2003. PMID: 12923522 Review. - Tissue-specific expression and dynamic organization of SR splicing factors in Arabidopsis.
Fang Y, Hearn S, Spector DL. Fang Y, et al. Mol Biol Cell. 2004 Jun;15(6):2664-73. doi: 10.1091/mbc.e04-02-0100. Epub 2004 Mar 19. Mol Biol Cell. 2004. PMID: 15034145 Free PMC article. - Nuclear speckles: molecular organization, biological function and role in disease.
Galganski L, Urbanek MO, Krzyzosiak WJ. Galganski L, et al. Nucleic Acids Res. 2017 Oct 13;45(18):10350-10368. doi: 10.1093/nar/gkx759. Nucleic Acids Res. 2017. PMID: 28977640 Free PMC article. Review. - SRSF1 regulates the assembly of pre-mRNA processing factors in nuclear speckles.
Tripathi V, Song DY, Zong X, Shevtsov SP, Hearn S, Fu XD, Dundr M, Prasanth KV. Tripathi V, et al. Mol Biol Cell. 2012 Sep;23(18):3694-706. doi: 10.1091/mbc.E12-03-0206. Epub 2012 Aug 1. Mol Biol Cell. 2012. PMID: 22855529 Free PMC article. - Disassembly of interchromatin granule clusters alters the coordination of transcription and pre-mRNA splicing.
Sacco-Bubulya P, Spector DL. Sacco-Bubulya P, et al. J Cell Biol. 2002 Feb 4;156(3):425-36. doi: 10.1083/jcb.200107017. Epub 2002 Feb 4. J Cell Biol. 2002. PMID: 11827980 Free PMC article.
Cited by
- Roles of lncRNAs related to the p53 network in breast cancer progression.
Song J, Cui Q, Gao J. Song J, et al. Front Oncol. 2024 Oct 16;14:1453807. doi: 10.3389/fonc.2024.1453807. eCollection 2024. Front Oncol. 2024. PMID: 39479021 Free PMC article. Review. - Identification and characterization of putative effectors from Plasmodiophora brassicae that suppress or induce cell death in Nicotiana benthamiana.
Zhan Z, Liu H, Yang Y, Liu S, Li X, Piao Z. Zhan Z, et al. Front Plant Sci. 2022 Sep 20;13:881992. doi: 10.3389/fpls.2022.881992. eCollection 2022. Front Plant Sci. 2022. PMID: 36204052 Free PMC article. - FTO levels affect RNA modification and the transcriptome.
Berulava T, Ziehe M, Klein-Hitpass L, Mladenov E, Thomale J, Rüther U, Horsthemke B. Berulava T, et al. Eur J Hum Genet. 2013 Mar;21(3):317-23. doi: 10.1038/ejhg.2012.168. Epub 2012 Aug 8. Eur J Hum Genet. 2013. PMID: 22872099 Free PMC article. - Biological Phase Separation and Biomolecular Condensates in Plants.
Emenecker RJ, Holehouse AS, Strader LC. Emenecker RJ, et al. Annu Rev Plant Biol. 2021 Jun 17;72:17-46. doi: 10.1146/annurev-arplant-081720-015238. Epub 2021 Mar 8. Annu Rev Plant Biol. 2021. PMID: 33684296 Free PMC article. Review. - Amyloids assemble as part of recognizable structures during oogenesis in Xenopus.
Hayes MH, Weeks DL. Hayes MH, et al. Biol Open. 2016 Jun 15;5(6):801-6. doi: 10.1242/bio.017384. Biol Open. 2016. PMID: 27215327 Free PMC article.
References
- Beck JS 1961. Variations in the morphological patterns of “autoimmune” nuclear fluorescence. Lancet 1: 1203–1205 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources