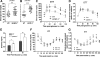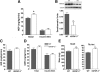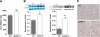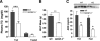Evidence of a role for insulin-like growth factor binding protein (IGFBP)-3 in metabolic regulation - PubMed (original) (raw)
Evidence of a role for insulin-like growth factor binding protein (IGFBP)-3 in metabolic regulation
P M Yamada et al. Endocrinology. 2010 Dec.
Abstract
IGF-binding protein (IGFBP)-3 is a metabolic regulator that has been shown to inhibit insulin-stimulated glucose uptake in murine models. This finding contrasts with epidemiological evidence of decreased serum IGFBP-3 in patients with type 2 diabetes. The purpose of this study was to clarify the role of IGFBP-3 in metabolism. Four-week-old male IGFBP-3(-/-) and control mice were subjected to a high-fat diet (HFD) for 12 wk. IGFBP-3(-/-) mice were heavier before the initiation of HFD and at the end of the study period. Resting metabolic rate was significantly decreased in knockout mice; however, respiratory exchange ratio was not significantly different. Fasting blood glucose and insulin levels were significantly elevated in IGFBP-3(-/-) mice. However, IGFBP-3(-/-) mice had relatively normal glucose tolerance because the relative glucose excursion over time was not different between the groups. During hyperinsulinemic clamps, IGFBP-3(-/-) mice had increased basal hepatic glucose production, but after insulin stimulation, no differences in hepatic glucose production were observed. A second cohort of older IGFBP-3(-/-) mice on HFD displayed unexpected evidence of hepatic steatosis. In summary, glucose tolerance and clamp testing indicate that IGFBP-3(-/-) mice preserve insulin sensitivity despite evidence of increased basal glucose turnover and hepatic steatosis. We provide evidence that genetic deletion of IGFBP-3 modulates hepatic carbohydrate and lipid metabolism.
Figures
Figure 1
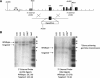
Targeted disruption of the Igfbp3 gene locus. A, Targeting strategy used to disrupt the Igfbp3 locus. Homologous recombination (represented by ×) between the targeting vector and the Igfbp3 gene results in the replacement of the first coding exon with the selection cassette. The _Acc_65I and _Nde_I restriction enzyme cut sites used in the Southern blot analysis are indicated. B, Southern hybridization indicating proper gene targeting in the embryonic stem cell clones. Using a 5′ external probe on _Acc_65I-digested genomic DNA produces a 20.2-kb WT band and a 14.6-kb targeted band. Using a 3′ external probe on _Nde_I-digested genomic DNA produces a 10.2-kb WT band and a 7.6-kb targeted band. Clone 2G6 was selected for blastocyst injections. Lex1 represents untransfected embryonic stem cell DNA.
Figure 2
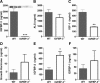
Circulating IGFBP-3 and related analytes. The 30-wk-old male mice were analyzed after HFD. A, Serum IGFBP-3 was measured using an in-house ELISA and immunoblotting (inset); B, ALS was measured using ELISA; C, reduced IGF-I levels in IGFBP-3−/− mice was expected response because IGFBP-3 dramatically extends the half-life of IGF-I; D, GH levels are not different; E, IGFBP-2 is significantly elevated in IGFBP-3−/− mice; F, IGFBP-1 was measured after a 5-h fast. *, P < 0.05; **, P < 0.01. Means ±
sem
are presented; n = 5–7 per group.
Figure 3
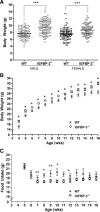
Body weight and food intake. A, Male and female IGFBP-3−/− and WT mice were weighed at weaning (3 wk old) (n = 90–130 per group); B, male IGFBP-3−/− are heavier than male WT mice at all time points using Holm’s sequential Bonferroni adjustment (n = 30–40 per group; HFD was initiated at 4 wk of age); C, male IGFBP-3−/− mice consumed less food at wk 7 and 9 when the significance level was adjusted with Holm’s sequential Bonferroni method. Using unadjusted significance levels (P < 0.05), IGFBP-3−/− mice had significantly lower food consumption from wk 7–10 and wk 12; n = 30–40. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Means ±
sem
are presented.
Figure 4
Basal glucose levels, GTT, and ITT. A, Fasting blood glucose was measured in male mice at 4 wk of age before HFD, after a 5-h fast; B, after 12 wk of HFD, fasting blood glucose levels doubled in IGFBP-3−/− mice; C, absolute blood glucose levels were significantly higher in IGFBP-3−/− mice; D, blood glucose was not different during the GTT when glucose levels were expressed relative to baseline levels (n = 10 per group); E, basal insulin concentrations were higher in IGFBP-3−/− mice, but no differences were observed 30 min after glucose injection (n = 10 per group); F, absolute blood glucose levels were not different during the ITT; G, blood glucose levels were reduced in IGFBP-3−/− mice at 150, 180, and 210 min after insulin injection when glucose levels were expressed relative to baseline during ITT, adjusted with Holm-Bonferroni adjustment (n = 5–6 per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Means ±
sem
are presented.
Figure 5
Hepatic insulin sensitivity. A, In 16-wk-old male mice after HFD, basal HGP is significantly elevated in IGFBP-3−/− mice, but no differences were observed during insulin stimulation (n = 7–8 per group); B, during insulin stimulation, PEPCK was suppressed in the KO compared with WT as detected with immunoblotting (inset) and densitometric analyses; C, glucose infusion rate was not significantly different (n = 7–8 per group); D, no differences in total and insulin-stimulated glucose disposal rate (n = 7–8 per group); E, 16-wk-old male IGFBP-3−/− mice have greater quadriceps (Quad) and tibialis anterior (Tib Ant) muscle mass in the fasted state (n = 8–11 per group). *, P < 0.05. Means ±
sem
are presented.
Figure 6
Hepatic lipid metabolism. A, The 16-wk-old IGFBP-3−/− male mice have heavier liver mass in the fasted state (n = 15–20 per group); B, FAS was up-regulated in IGFBP-3−/− mice under insulin stimulation as shown with immunoblotting (inset) and densitometric analyses, and p-JNK was up-regulated in IGFBP-3−/− mice as shown with immunoblotting and densitometric analyses in the fed state; C, greater hepatic oil red O staining in 30-wk-old male IGFBP-3−/− mice after HFD. Magnification, ×100. *, P < 0.05; **, P < 0.01. Means ±
sem
are presented.
Figure 7
Adipose tissue, triglycerides, and adiponectin. A, In 16-wk-old IGFBP-3−/− male mice, plasma triglycerides (TG) were reduced in the fed state but not different in the fasted state (n = 5–7 per group); B, reduced WAT mass in the IGFBP-3−/− group in a fed state (n = 15–20 per group); C, IGFBP-3−/− mice have reduced adiponectin (ADN) levels as measured with ELISA in both fed and fasted states (n = 5 per group) and as measured with immunoblotting in fed plasma (inset). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Means ±
sem
are presented.
Similar articles
- Endogenous interleukin-10 protects against hepatic steatosis but does not improve insulin sensitivity during high-fat feeding in mice.
den Boer MA, Voshol PJ, Schröder-van der Elst JP, Korsheninnikova E, Ouwens DM, Kuipers F, Havekes LM, Romijn JA. den Boer MA, et al. Endocrinology. 2006 Oct;147(10):4553-8. doi: 10.1210/en.2006-0417. Epub 2006 May 18. Endocrinology. 2006. PMID: 16709607 - Effects of milk replacer composition on selected blood metabolites and hormones in preweaned Holstein heifers.
Daniels KM, Hill SR, Knowlton KF, James RE, McGilliard ML, Akers RM. Daniels KM, et al. J Dairy Sci. 2008 Jul;91(7):2628-40. doi: 10.3168/jds.2007-0859. J Dairy Sci. 2008. PMID: 18565922 - Pappa2 deletion alters IGFBPs but has little effect on glucose disposal or adiposity.
Christians JK, Bath AK, Amiri N. Christians JK, et al. Growth Horm IGF Res. 2015 Oct;25(5):232-9. doi: 10.1016/j.ghir.2015.07.001. Epub 2015 Jul 7. Growth Horm IGF Res. 2015. PMID: 26164771 - Role of insulin-like growth factor binding protein-3 in glucose and lipid metabolism.
Kim HS. Kim HS. Ann Pediatr Endocrinol Metab. 2013 Mar;18(1):9-12. doi: 10.6065/apem.2013.18.1.9. Epub 2013 Mar 31. Ann Pediatr Endocrinol Metab. 2013. PMID: 24904844 Free PMC article. Review.
Cited by
- Mouse models of growth hormone insensitivity.
Young J, Bell S, Qian Y, Hyman C, Berryman DE. Young J, et al. Rev Endocr Metab Disord. 2021 Mar;22(1):17-29. doi: 10.1007/s11154-020-09600-6. Epub 2020 Oct 10. Rev Endocr Metab Disord. 2021. PMID: 33037595 Free PMC article. Review. - Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents.
Chin YP, Keni J, Wan J, Mehta H, Anene F, Jia Y, Lue YH, Swerdloff R, Cobb LJ, Wang C, Cohen P. Chin YP, et al. Endocrinology. 2013 Oct;154(10):3739-44. doi: 10.1210/en.2012-2004. Epub 2013 Jul 8. Endocrinology. 2013. PMID: 23836030 Free PMC article. - Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus.
Rojas-Rodriguez R, Lifshitz LM, Bellve KD, Min SY, Pires J, Leung K, Boeras C, Sert A, Draper JT, Corvera S, Moore Simas TA. Rojas-Rodriguez R, et al. Diabetologia. 2015 Sep;58(9):2106-14. doi: 10.1007/s00125-015-3662-0. Epub 2015 Jun 13. Diabetologia. 2015. PMID: 26067361 Free PMC article. - Combination of Recreational Soccer and Caloric Restricted Diet Reduces Markers of Protein Catabolism and Cardiovascular Risk in Patients with Type 2 Diabetes.
Vieira de Sousa M, Fukui R, Krustrup P, Dagogo-Jack S, Rossi da Silva ME. Vieira de Sousa M, et al. J Nutr Health Aging. 2017;21(2):180-186. doi: 10.1007/s12603-015-0708-4. J Nutr Health Aging. 2017. PMID: 28112773 Clinical Trial. - The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes.
Haywood NJ, Slater TA, Matthews CJ, Wheatcroft SB. Haywood NJ, et al. Mol Metab. 2019 Jan;19:86-96. doi: 10.1016/j.molmet.2018.10.008. Epub 2018 Oct 24. Mol Metab. 2019. PMID: 30392760 Free PMC article. Review.
References
- Guler HP, Zapf J, Schmid C, Froesch ER 1989 Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 121:753–758 - PubMed
- Chan SS, Twigg SM, Firth SM, Baxter RC 2005 Insulin-like growth factor binding protein-3 leads to insulin resistance in adipocytes. J Clin Endocrinol Metab 90:6588–6595 - PubMed
- Yamada PM, Lee KW 2009 Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol 296:C954–C976 - PubMed
- Bang P, Brismar K, Rosenfeld RG 1994 Increased proteolysis of insulin-like growth factor-binding protein-3 (IGFBP-3) in noninsulin-dependent diabetes mellitus serum, with elevation of a 29-kilodalton (kDa) glycosylated IGFBP-3 fragment contained in the approximately 130- to 150-kDa ternary complex. J Clin Endocrinol Metab 78:1119–1127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2P30DK063491/DK/NIDDK NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- DK73227/DK/NIDDK NIH HHS/United States
- DK78760/DK/NIDDK NIH HHS/United States
- R21 DK073227/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous