Inner/Outer nuclear membrane fusion in nuclear pore assembly: biochemical demonstration and molecular analysis - PubMed (original) (raw)
Inner/Outer nuclear membrane fusion in nuclear pore assembly: biochemical demonstration and molecular analysis
Boris Fichtman et al. Mol Biol Cell. 2010 Dec.
Abstract
Nuclear pore complexes (NPCs) are large proteinaceous channels embedded in double nuclear membranes, which carry out nucleocytoplasmic exchange. The mechanism of nuclear pore assembly involves a unique challenge, as it requires creation of a long-lived membrane-lined channel connecting the inner and outer nuclear membranes. This stabilized membrane channel has little evolutionary precedent. Here we mapped inner/outer nuclear membrane fusion in NPC assembly biochemically by using novel assembly intermediates and membrane fusion inhibitors. Incubation of a Xenopus in vitro nuclear assembly system at 14°C revealed an early pore intermediate where nucleoporin subunits POM121 and the Nup107-160 complex were organized in a punctate pattern on the inner nuclear membrane. With time, this intermediate progressed to diffusion channel formation and finally to complete nuclear pore assembly. Correct channel formation was blocked by the hemifusion inhibitor lysophosphatidylcholine (LPC), but not if a complementary-shaped lipid, oleic acid (OA), was simultaneously added, as determined with a novel fluorescent dextran-quenching assay. Importantly, recruitment of the bulk of FG nucleoporins, characteristic of mature nuclear pores, was not observed before diffusion channel formation and was prevented by LPC or OA, but not by LPC+OA. These results map the crucial inner/outer nuclear membrane fusion event of NPC assembly downstream of POM121/Nup107-160 complex interaction and upstream or at the time of FG nucleoporin recruitment.
Figures
Figure 1.
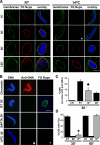
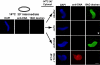
Cold temperature produces a novel intermediate in NPC assembly at 30 min that lacks FG Nups. (A) The progression of nuclear envelope assembly is slowed down at 14°C. Nuclear assembly reactions containing chromatin, cytosol, and membranes were set up at different temperatures. Reactions were stopped at the indicated time points and analyzed by confocal microscopy after direct immunofluorescence with the Alexa568-labeled anti-FG Nup antibody mAb414 (red). Nuclear membranes were visualized with the fluorescent membrane dye DHCC (green), and chromatin was stained with DAPI (blue). Scale bar, 10 μm. (B) A DNA antibody exclusion assay was used to determine the formation of closed nuclear envelopes (CNE). For this, nuclear assembly reactions containing chromatin, cytosol and membranes were set up and incubated for 30 min at room temperature (RT), for 30 min at 14°C, and for 30 min at RT in the presence of BAPTA before fixation. Alexa568-labeled anti-DNA antibody (red) and Alexa488-labeled anti-FG Nup antibody (green) were added and the nuclei examined. Chromatin plus cytosol (no membranes) served as a positive control for anti-DNA antibody binding (top panel). Scale bar, 10 μm. (C) The formation of CNE was quantified for at least 100 randomly chosen structures in three independent experiments. (D) The percentage of CNE containing FG Nups, detected as a green rim with Alexa488-labeled anti-FG Nup antibody at 30 min and 60 min in three different experiments was also plotted. Errors bars indicate the SD. Nuclei reconstituted in equivalent conditions of 30 min 14°C are marked with a diamond for anti-DNA antibody (♦) and an asterisk (*) for anti-FG Nup antibody in panels A–D.
Figure 2.
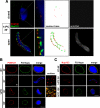
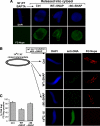
The 30-min cold intermediate contains punctate POM121 and Nup133 on the inner face of the nuclear envelope. (A) A nuclear assembly reaction was incubated for 30 min at 14°C and analyzed by confocal microscopy after direct immunofluorescence on ice with Alexa568-anti-POM121 (red) and Alexa488-anti-Nup133 (green) antibodies (left panels). The staining of the same nuclear intermediates with Alexa647-anti-DNA antibody (gray) is shown in the right panels; this latter staining was used to assess whether an individual nucleus contained a fully closed (top panels) or still open (bottom panels) nuclear envelope. Merged images of POM121/Nup133/DAPI stain are shown at normal (left panels) and high (adjacent panels) magnification. We note that POM121 and Nup133 could only be stained in intermediates where the nuclear envelope was open (i.e., accessible to anti-DNA antibody). A contour trace of the POM121 and Nup133 staining is shown in the 3rd set of panels, allowing comparison of their localization. Scale bar, 10 μm. (B) Nuclei with accessible interiors were analyzed by direct immunofluorescence with Alexa568-anti-POM121 (red) and Alexa488-anti-FG Nup (green) antibodies. The merged images (yellow) are shown at the right. Chromatin was stained with DAPI (blue). Scale bar, 10 μm. Individual NPCs were separately visualized at high magnification on the surface of 14°C nuclear intermediates assembled for 30 min and 60 min (surface). Scale bar, 1 μm. (C) The assembly reactions were analyzed in parallel with Alexa568-anti-Nup133 (red) and Alexa488-anti-FG Nup (green) antibodies. Scale bar, 10 μm.
Figure 3.
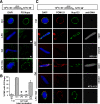
The 30-min cold intermediate containing POM121 and the Nup107-160 complex lacks diffusion channels. (A) An anti-DNA antibody exclusion assay was used to assess the formation of completely enclosed nuclear envelopes. Nuclei were assembled at room temperature for 60 min with chromatin, membranes, and either control cytosol, GTPγS-treated cytosol, or BAPTA-treated cytosol. Control nuclei were either visualized alone (Ctrl) or permeabilized with Triton X-100 (TX100) to allow entry of anti-DNA antibody. Positive labeling of the chromatin with Alexa568-anti-DNA antibody in the GTPγS nuclei indicated that a fully closed NE did not form under this condition; absence of anti-DNA antibody staining for Control and BAPTA nuclei indicated that a fully closed NE formed in these conditions. Scale bar, 10 μm. The percentage of nuclei showing a lack of DNA antibody staining in their nuclear interior (% CNE) was quantified for ∼100 randomly chosen nuclear intermediates each in three independent experiments, as shown in the histogram. Error bars indicate SD. (B) The anti-Alexa488 quenching assay described in Supplemental Figure 4 was used to determine the presence of 10-kDa competent diffusion channels in closed nuclear envelopes reconstituted for 15 min and 30 min at 14°C. The 15 min, 14°C intermediates were not fully membrane enclosed, as they showed anti-DNA staining (red) and quenching by anti-Alexa488 antibody (black) (top panels). By 30 min at 14°C, many intact nuclei had formed, as judged by a lack of DNA staining, but these showed no entry of Alexa488-10 kDa dextran indicating no diffusion channels (middle panels). In contrast, by 30 min at room temperature, the nuclei were intact (no anti-DNA staining) but had developed nuclear pores capable of allowing Alexa488-dextran entry, enabling its protection from the quenching antibody (lower panels). (C) The percentage of 14°C 30 min enclosed nuclear intermediates (i.e., with no anti-DNA staining) that showed diffusion of Alexa488-10 kDa or Alexa488-3 kDa dextrans was measured in three different experiments each and plotted. We found that the great majority of enclosed 14°C 30 min nuclear intermediates did not show diffusion channels. Errors bars indicate the SD.
Figure 4.
Channel formation is a late event in NPC assembly. To ask whether the nuclear intermediates that did not show diffusion channels by 30 min at 14°C were competent to develop such channels, the diffusion assay in Figure 3B was performed for both 30 min and 60 min at 14°C. Only nuclei showing closed nuclear envelopes (CNE) according to the anti-DNA antibody staining assay were analyzed for 3-kDa dextran uptake and were quantitated. At 30 min at 14°C, only 4% of intact nuclear intermediates showed internal Alexa488-3 kDa dextran. By 60 min at 14°C, however, 100% of the intact nuclear intermediates showed internal protected Alexa488-3 kDa dextran. Scale bars, 10 μm.
Figure 5.
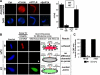
LPC blocks diffusion channel formation. Nuclei were assembled for 30 min at 14°C, and the preparation was verified for the absence of diffusion channels, as in Figure 3B, with Alexa488-10 kDa dextran (left panels). The reaction was then supplemented with buffer, LPC, or LPC plus OA, followed by incubation for 90 min at 14°C to assess the affect of LPC on channel formation. The nuclei were assayed for channel formation with Alexa488-10 kDa dextran. A block to channel formation was observed with LPC (asterisk). When LPC and OA were added together, however, diffusion of Alexa488-10 kDa dextran into the nuclei was now observed. Control nuclei assembled at room temperature for 60 min that were the treated with Triton X-100 before the addition of antibodies served as a positive control for the anti-DNA antibody assay and for the dextran quenching assay (bottom panels). Scale bar, 10 μm.
Figure 6.
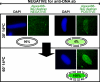
The cold intermediate contains all the membrane proteins required for NPC assembly. To determine whether the 14°C 30-min cold intermediate is able to go on to form nuclear pores without the addition of extra membranes, WTαSNAP was added as a vesicle–vesicle fusion inhibitor. Δ80NαSNAP served as a negative control. (A) We first looked at BAPTA-arrested pore-free nuclei which had been formed at room temperature for 30 min (left panels). Because neither SNAP protein is membrane permeable, we expected that WTαSNAP should inhibit addition of new vesicles to the forming nuclei, but could not inhibit inner-outer nuclear membrane fusion, thus permitting nuclear pore formation. Consistent with this, 30-min BAPTA-arrested pore-free nuclei which had been formed at room temperature, when diluted 1:10 into cytosol supplemented with buffer, WTαSNAP, or Δ80NαSNAP and incubated for a further 30 min, all became FG Nup–positive, as detected by Alexa488-anti-FG Nup antibody (green). (B) We performed the same type of experiment with nuclei reconstituted at 14°C for 30 min, at which time the reaction contained ∼50% closed, FG-negative nuclear intermediates. (Because of SNAP inhibition of vesicle–vesicle fusion, the open intermediates did not progress further.) The 14°C 30 min nuclei were diluted 1:10 into cytosol containing buffer, WTαSNAP (47 μM), or Δ80NαSNAP (47 μM) and incubated for a further 30 min at 14°C. Reactions were stopped on ice, fixed, and incubated on ice with Alexa488-anti-DNA (green) and Alexa568-anti-FG Nup (red) antibodies. All three reactions produced abundant anti-DNA–negative, FG-Nup–positive nuceli (red). Nuclear intermediates which had been assembled for 15 min at 14°C served as a negative control for the anti-FG Nup antibody (0%) and a positive control for the anti-DNA antibody (100%). A representative nucleus is shown in the bottom panels. The FG Nup–positive nuclei in B were quantified for each of three replicate experiments and the average percentage containing FG Nups is plotted in C. Errors bars indicate the SD.
Figure 7.
The fusion inhibitors LPC and OA individually prevent FG Nup assembly in the 30 min cold intermediate. To ask whether membrane fusion inhibitors prevent formation of mature nuclear pores in the 30-min cold intermediate, intermediates were first formed at 14°C for 30 min and verified with Alexa647-anti-DNA and Alexa488-anti-FG Nup antibodies. Approximately 95% of the nuclei showed no FG Nup staining (B, left column). (A) At this point LPC, OA (final concentration 600 μM each), or a mix of both were added and the reactions were incubated at 14°C for an additional 90 min. The reactions were stopped on ice, fixed with 2.5% paraformaldehyde for 30 min on ice, followed by incubation for 30 min on ice with Alexa488-anti-FG Nup antibody (green). LPC or OA blocked FG recruitment (asterisks). (B) The data in A are quantitated, and error bars indicate SD. (C) In parallel, an aliquot of the nuclei in the fixed reactions from A were stained with a mixture of Alexa568-anti-POM121 (red), Alexa488-anti-Nup133 (green), and Alexa674-anti-DNA antibodies (gray). In A, LPC- or OA-treated nuclei at 120-min total incubation showed little FG Nup staining (A and B, asterisks), indicating no progression to an FG Nup–positive state. However, when LPC and OA were added together (A, B, and C), nuclear assembly occurred normally in that the majority of the 120 min closed nuclei showed POM121, Nup133, and FG Nup staining (LPC+OA).
Figure 8.
A schematic model for inner/outer nuclear membrane fusion in the early steps of vertebrate NPC assembly. In previous studies, ELYS was shown to mediate the binding of the Nup107-160 complex to sites of AT-rich chromatin (blue) in early nuclear assembly. Simultaneous with this ER-derived membranes are recruited to the rim of developing nuclei. The data presented here argues that next: (1) the Nup107-160 complex (detected by anti-Nup133 antibody) interacts with POM121 at the inner nuclear membrane in an early nuclear intermediate (30 min, 14°C). Bending of the membranes toward one another would theoretically occur at or near this time. (2) This is followed by fusion of the inner and outer nuclear membranes to form a diffusion channel. (3) This fusion is simultaneous with or precedes recruitment of the bulk of FG nucleoporins (gold) and assembly of the mature nuclear pore, which involves expansion of the initial channel and its stabilization. In pioneering membrane-membrane fusion studies, progression to hemifusion was seen to be typically inhibited by cone-shaped lipids, such as LPC; here LPC was found to indeed block channel formation and FG Nup assembly in nuclear intermediates. Progression beyond hemifusion to full fusion has been observed to be inhibited by inverted cone-shaped lipids, such as OA; here we found that OA also blocks mature nuclear pore assembly. When added together LPC and OA are known to geometrically neutralize one another, and we observed that LPC+OA allow channel formation, i.e., inner/outer membrane fusion. (It is important to note that we do not believe that a completely sealed nuclear envelope is required before nuclear pores can form, but possession of such a sealed intermediate allowed us to distinguish and analyze the channel formation step of nuclear pore assembly.)
Similar articles
- NEP-A and NEP-B both contribute to nuclear pore formation in Xenopus eggs and oocytes.
Salpingidou G, Rzepecki R, Kiseleva E, Lyon C, Lane B, Fusiek K, Golebiewska A, Drummond S, Allen T, Ellis JA, Smythe C, Goldberg MW, Hutchison CJ. Salpingidou G, et al. J Cell Sci. 2008 Mar 1;121(Pt 5):706-16. doi: 10.1242/jcs.019968. Epub 2008 Feb 12. J Cell Sci. 2008. PMID: 18270266 - POM121 and Sun1 play a role in early steps of interphase NPC assembly.
Talamas JA, Hetzer MW. Talamas JA, et al. J Cell Biol. 2011 Jul 11;194(1):27-37. doi: 10.1083/jcb.201012154. Epub 2011 Jul 4. J Cell Biol. 2011. PMID: 21727197 Free PMC article. - Nup153 Recruits the Nup107-160 Complex to the Inner Nuclear Membrane for Interphasic Nuclear Pore Complex Assembly.
Vollmer B, Lorenz M, Moreno-Andrés D, Bodenhöfer M, De Magistris P, Astrinidis SA, Schooley A, Flötenmeyer M, Leptihn S, Antonin W. Vollmer B, et al. Dev Cell. 2015 Jun 22;33(6):717-28. doi: 10.1016/j.devcel.2015.04.027. Epub 2015 Jun 4. Dev Cell. 2015. PMID: 26051542 - Nuclear pore biogenesis into an intact nuclear envelope.
Doucet CM, Hetzer MW. Doucet CM, et al. Chromosoma. 2010 Oct;119(5):469-77. doi: 10.1007/s00412-010-0289-2. Epub 2010 Aug 19. Chromosoma. 2010. PMID: 20721671 Review. - Structure and Assembly of the Nuclear Pore Complex.
Hampoelz B, Andres-Pons A, Kastritis P, Beck M. Hampoelz B, et al. Annu Rev Biophys. 2019 May 6;48:515-536. doi: 10.1146/annurev-biophys-052118-115308. Epub 2019 Apr 3. Annu Rev Biophys. 2019. PMID: 30943044 Review.
Cited by
- Nuclear size, nuclear pore number and cell cycle.
Maeshima K, Iino H, Hihara S, Imamoto N. Maeshima K, et al. Nucleus. 2011 Mar-Apr;2(2):113-8. doi: 10.4161/nucl.2.2.15446. Nucleus. 2011. PMID: 21738834 Free PMC article. - In vitro assembly of nuclear envelope in tobacco cultured cells.
Tamura K, Ueda H, Hara-Nishimura I. Tamura K, et al. Nucleus. 2021 Dec;12(1):82-89. doi: 10.1080/19491034.2021.1930681. Nucleus. 2021. PMID: 34030583 Free PMC article. - Nuclear envelope impairment is facilitated by the herpes simplex virus 1 Us3 kinase.
Wild P, Leisinger S, de Oliveira AP, Doehner J, Schraner EM, Fraevel C, Ackermann M, Kaech A. Wild P, et al. F1000Res. 2019 Feb 18;8:198. doi: 10.12688/f1000research.17802.1. eCollection 2019. F1000Res. 2019. PMID: 31249678 Free PMC article. - Minimization of extracellular space as a driving force in prokaryote association and the origin of eukaryotes.
Hooper SL, Burstein HJ. Hooper SL, et al. Biol Direct. 2014 Nov 18;9(1):24. doi: 10.1186/1745-6150-9-24. Biol Direct. 2014. PMID: 25406691 Free PMC article. - An unresolved LINC in the nuclear envelope.
Torbati M, Lele TP, Agrawal A. Torbati M, et al. Cell Mol Bioeng. 2016 Jun;9(2):252-257. doi: 10.1007/s12195-016-0431-1. Epub 2016 Feb 18. Cell Mol Bioeng. 2016. PMID: 27330571 Free PMC article.
References
- Anderson D. J., Hetzer M. W. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat. Cell Biol. 2007;9:1160–1166. - PubMed
- Antonin W., Ellenberg J., Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. - PubMed
- Antonin W., Franz C., Haselmann U., Antony C., Mattaj I. W. The integral membrane nucleoporin pom121 functionally links nuclear pore complex assembly and nuclear envelope formation. Mol. Cell. 2005;17:83–92. - PubMed
- Baur T., Ramadan K., Schlundt A., Kartenbeck J., Meyer H. H. NSF- and SNARE-mediated membrane fusion is required for nuclear envelope formation and completion of nuclear pore complex assembly in Xenopus laevis egg extracts. J. Cell Sci. 2007;120:2895–2903. - PubMed
- Beck M., Forster F., Ecke M., Plitzko J. M., Melchior F., Gerisch G., Baumeister W., Medalia O. Nuclear pore complex structure and dynamics revealed by cryoelectron tomography. Science. 2004;306:1387–1390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous