Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro - PubMed (original) (raw)
Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro
Nick De Regge et al. PLoS One. 2010.
Abstract
Background: Several alphaherpesviruses, including herpes simplex virus 1 (HSV-1) and pseudorabies virus (PRV), establish lifelong latency in neurons of the trigeminal ganglion (TG). Although it is thought that efficient establishment of alphaherpesvirus latency is based on a subtle interplay between virus, neurons and the immune system, it is not clear which immune components are of major importance for the establishment of latency.
Methodology/principal findings: Here, using an in vitro model that enables a natural route of infection, we show that interferon alpha (IFNalpha) has the previously uncharacterized capacity to induce a quiescent HSV-1 and PRV infection in porcine TG neurons that shows strong similarity to in vivo latency. IFNalpha induced a stably suppressed HSV-1 and PRV infection in TG neurons in vitro. Subsequent treatment of neurons containing stably suppressed virus with forskolin resulted in reactivation of both viruses. HSV and PRV latency in vivo is often accompanied by the expression of latency associated transcripts (LATs). Infection of TG neurons with an HSV-1 mutant expressing LacZ under control of the LAT promoter showed activation of the LAT promoter and RT-PCR analysis confirmed that both HSV-1 and PRV express LATs during latency in vitro.
Conclusions/significance: These data represent a unique in vitro model of alphaherpesvirus latency and indicate that IFNalpha may be a driving force in promoting efficient latency establishment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
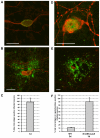
Figure 1. Productive replication of PRV and HSV-1 in porcine TG neurons.
Confocal images of TG neuronal cultures in the inner chamber at 24hpi with PRV (A,B) and 48hpi with HSV-1 (D,E) stained for neurofilament (red) and late viral antigens (green) (bar = 50 µm). Percentage of neurons with axons growing out to the outer chamber that show viral antigens at 24hpi with PRV (C) and 48hpi with wt HSV-1 (F, left bar) and beta-galactosidase activity at 24hpi with SΔUS5-LacZ HSV-1 (F, right bar). Data represent the mean ± s.e.m. of three independent experiments.
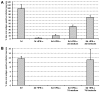
Figure 2. IFNalpha induces a reactivatable, latent PRV and HSV-1 infection in porcine TG neurons.
Percentage infected neurons that are late viral antigen positive at 1, 5 and 8dpi with PRV (A) and at 2, 5 and 12dpi with HSV-1 (B) in the presence or absence of 500 U/ml IFNalpha. For the neurons fixed at 8dpi with PRV and 12dpi with HSV-1, medium containing IFNalpha was washed out at 5dpi and replaced with new culture medium or new culture medium supplemented with forskolin (200 µM). Data represent the mean ± s.e.m. of three independent experiments.
Figure 3. PRV and HSV-1 express LATs during in vitro latency.
(A,B) RT-PCR analysis of actin and viral immediate early (IE180 and ICP0), late (gB and gD) and LAT transcript RNA isolated from neuronal cultures that were either mock infected, productively infected with PRV (A, 1dpi) or HSV-1 (B, 2dpi), or latently infected with PRV (A, 5dpi with IFNalpha) or HSV-1 (B, 9dpi, 4 days post IFNalpha withdrawal). For each condition three different samples were analyzed and representative gels are shown. For HSV-1, two samples of 9dpi, 4 days post IFNalpha withdrawal are shown, one without and one with detectable ICP0 transcript expression. Specific bands are marked with a black arrowhead. (C) Percentage of infected neurons positive for LAT promoter-driven beta-galactosidase at 2 and 5dpi with HSV-1 LbetaA in the presence or absence of 500 U/ml IFNalpha. Data represent the mean ± s.e.m. of three independent experiments. (D) Light microscopic images of uniform (i,ii) and focal (iii) LAT promoter-driven beta-galactosidase distribution during the acute stage (2dpi without IFNalpha, i, ii) or the latent stage (5dpi with IFNalpha, iii) of infection with HSV-1 LbetaA. Arrows point to infected non-neuronal cells (i), dashed line marks contour of neuronal cell body in (iii) (bar = 20 µm).
Similar articles
- Effects of interferon on immediate-early mRNA and protein levels in sensory neuronal cells infected with herpes simplex virus type 1 or pseudorabies virus.
Van Opdenbosch N, De Regge N, Van Poucke M, Peelman L, Favoreel HW. Van Opdenbosch N, et al. Vet Microbiol. 2011 Sep 28;152(3-4):401-6. doi: 10.1016/j.vetmic.2011.05.010. Epub 2011 May 13. Vet Microbiol. 2011. PMID: 21641126 - A 2.5-kilobase deletion containing a cluster of nine microRNAs in the latency-associated-transcript locus of the pseudorabies virus affects the host response of porcine trigeminal ganglia during established latency.
Mahjoub N, Dhorne-Pollet S, Fuchs W, Endale Ahanda ML, Lange E, Klupp B, Arya A, Loveland JE, Lefevre F, Mettenleiter TC, Giuffra E. Mahjoub N, et al. J Virol. 2015 Jan;89(1):428-42. doi: 10.1128/JVI.02181-14. Epub 2014 Oct 15. J Virol. 2015. PMID: 25320324 Free PMC article. - Modulation of Voltage-Gated Sodium Channel Activity in Human Dorsal Root Ganglion Neurons by Herpesvirus Quiescent Infection.
Zhang Q, Martin-Caraballo M, Hsia SV. Zhang Q, et al. J Virol. 2020 Jan 17;94(3):e01823-19. doi: 10.1128/JVI.01823-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694955 Free PMC article. - The latency-associated gene of herpes simplex virus type 1 (HSV-1) interferes with superinfection by HSV-1.
Mador N, Panet A, Steiner I. Mador N, et al. J Neurovirol. 2002 Dec;8 Suppl 2:97-102. doi: 10.1080/13550280290167920. J Neurovirol. 2002. PMID: 12491159 Review. - A Comparison of Pseudorabies Virus Latency to Other α-Herpesvirinae Subfamily Members.
Chen J, Li G, Wan C, Li Y, Peng L, Fang R, Peng Y, Ye C. Chen J, et al. Viruses. 2022 Jun 24;14(7):1386. doi: 10.3390/v14071386. Viruses. 2022. PMID: 35891367 Free PMC article. Review.
Cited by
- A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro.
Bertke AS, Swanson SM, Chen J, Imai Y, Kinchington PR, Margolis TP. Bertke AS, et al. J Virol. 2011 Jul;85(13):6669-77. doi: 10.1128/JVI.00204-11. Epub 2011 Apr 20. J Virol. 2011. PMID: 21507969 Free PMC article. - The Swine IFN System in Viral Infections: Major Advances and Translational Prospects.
Razzuoli E, Armando F, De Paolis L, Ciurkiewicz M, Amadori M. Razzuoli E, et al. Pathogens. 2022 Jan 27;11(2):175. doi: 10.3390/pathogens11020175. Pathogens. 2022. PMID: 35215119 Free PMC article. Review. - Latent versus productive infection: the alpha herpesvirus switch.
Koyuncu OO, MacGibeny MA, Enquist LW. Koyuncu OO, et al. Future Virol. 2018 May;13(6):431-443. doi: 10.2217/fvl-2018-0023. Epub 2018 May 22. Future Virol. 2018. PMID: 29967651 Free PMC article. Review. - Kaposi's sarcoma-associated herpesvirus vIRF2 protein utilizes an IFN-dependent pathway to regulate viral early gene expression.
Koch S, Damas M, Freise A, Hage E, Dhingra A, Rückert J, Gallo A, Kremmer E, Tegge W, Brönstrup M, Brune W, Schulz TF. Koch S, et al. PLoS Pathog. 2019 May 6;15(5):e1007743. doi: 10.1371/journal.ppat.1007743. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31059555 Free PMC article.
References
- Croen KD, Ostrove JM, Dragovic LJ, Smialek JE, Straus SE. Latent herpes simplex virus in human trigeminal ganglia. Detection of an immediate early gene “anti-sense” transcript by in situ hybridization. N Engl J Med. 1987;317:1427–1432. - PubMed
- Gutekunst DE, Pirtle EC, Miller LD, Stewart WC. Isolation of pseudorabies virus from trigeminal ganglia of a latently infected sow. Am J Vet Res. 1980;41:1315–1316. - PubMed
- Decman V, Freeman ML, Kinchington PR, Hendricks RL. Immune control of HSV-1 latency. Viral Immunol. 2005;18:466–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous