Helicobacter pylori and gastric cancer: factors that modulate disease risk - PubMed (original) (raw)
Review
Helicobacter pylori and gastric cancer: factors that modulate disease risk
Lydia E Wroblewski et al. Clin Microbiol Rev. 2010 Oct.
Abstract
Helicobacter pylori is a gastric pathogen that colonizes approximately 50% of the world's population. Infection with H. pylori causes chronic inflammation and significantly increases the risk of developing duodenal and gastric ulcer disease and gastric cancer. Infection with H. pylori is the strongest known risk factor for gastric cancer, which is the second leading cause of cancer-related deaths worldwide. Once H. pylori colonizes the gastric environment, it persists for the lifetime of the host, suggesting that the host immune response is ineffective in clearing this bacterium. In this review, we discuss the host immune response and examine other host factors that increase the pathogenic potential of this bacterium, including host polymorphisms, alterations to the apical-junctional complex, and the effects of environmental factors. In addition to host effects and responses, H. pylori strains are genetically diverse. We discuss the main virulence determinants in H. pylori strains and the correlation between these and the diverse clinical outcomes following H. pylori infection. Since H. pylori inhibits the gastric epithelium of half of the world, it is crucial that we continue to gain understanding of host and microbial factors that increase the risk of developing more severe clinical outcomes.
Figures
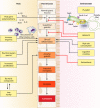
FIG. 1.
Multifactorial pathway leading to gastric carcinoma. Many host, bacterial, and environmental factors act in combination to contribute to the precancerous cascade leading to development of gastric cancer.
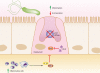
FIG. 2.
Relationships between H. pylori, inflammation, and acid secretion. H. pylori infection can reduce acid secretion and increase inflammation via multiple intermediates. Increased production of IL-1β and TNF-α from inflammatory cells inhibits acid secretion from parietal cells. Acid secretion is also inhibited by repression of H+K+ ATPase α-subunit promoter activity, in addition to VacA-induced proteolysis of ezrin.
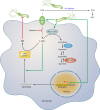
FIG. 3.
Pathways involved in regulation of macrophage iNOS synthesis and NO production in response to H. pylori. The translation of iNOS protein depends on the availability of
l
-arginine (
l
-Arg). Pathogenic mechanisms that inhibit
l
-Arg availability for iNOS include (i) the consumption of extracellular
l
-Arg by H. pylori itself, through its bacterial arginase activity; (ii) the upregulation of macrophage arginase II, which depletes intracellular
l
-Arg; and (iii) induction of ODC that generates the polyamine spermine, which blocks uptake of
l
-Arg into macrophages by CAT2. The resulting effect is limitation of iNOS protein synthesis and NO production, despite high levels of iNOS mRNA. Arginase and ODC are novel targets for therapeutic intervention to enhance antimicrobial NO production and hence reduce persistent colonization that leads to chronic inflammation and cancer risk.
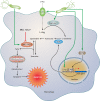
FIG. 4.
Mechanism of macrophage apoptosis caused by H. pylori. This pathway is dependent on the activities of the enzymes arginase II, ODC, and SMO. Induction of arginase II enhances synthesis of
l
-ornithine, which is converted into polyamines by ODC via a process that requires both H. pylori activation of the ODC promoter and c-Myc as a transcriptional enhancer. Production of the polyamine spermine provides a substrate for SMO, which is also upregulated by H. pylori. SMO generates H2O2, which causes mitochondrial membrane depolarization, cytochrome c release from mitochondria to the cytosol, and caspase-3 activation, followed by apoptosis. Induction of macrophage apoptosis leads to impairment of mucosal immunity to H. pylori, chronic inflammation, and cancer risk (48, 50, 116).
FIG. 5.
Dysregulation of the apical-junctional complex by H. pylori. H. pylori preferentially adheres to the apical-junctional complex of epithelial cells and alters localization of apical-junctional component proteins, disrupts epithelial barrier function, cell adhesion, and cell polarity, and induces an invasive phenotype. Translocated CagA interacts with PAR1, preventing phosphorylation of PAR1 by blocking PAR1 kinase activity, which culminates in disruption of the tight junction. In addition, functional urease activity can disrupt the tight junction via a mechanism involving MLC phosphorylation, which can be regulated by MLCK and Rho kinase. VacA can also increase tight junction permeability to low-molecular-weight molecules and ions.
Similar articles
- Host pathogen interactions in Helicobacter pylori related gastric cancer.
Chmiela M, Karwowska Z, Gonciarz W, Allushi B, Stączek P. Chmiela M, et al. World J Gastroenterol. 2017 Mar 7;23(9):1521-1540. doi: 10.3748/wjg.v23.i9.1521. World J Gastroenterol. 2017. PMID: 28321154 Free PMC article. Review. - Helicobacter pylori infection and gastric cancer: host, bug, environment, or all three?
Menaker RJ, Sharaf AA, Jones NL. Menaker RJ, et al. Curr Gastroenterol Rep. 2004 Dec;6(6):429-35. doi: 10.1007/s11894-004-0063-9. Curr Gastroenterol Rep. 2004. PMID: 15527671 Review. - Helicobacter and gastric malignancies.
Ferreira AC, Isomoto H, Moriyama M, Fujioka T, Machado JC, Yamaoka Y. Ferreira AC, et al. Helicobacter. 2008 Oct;13 Suppl 1(Suppl 1):28-34. doi: 10.1111/j.1523-5378.2008.00633.x. Helicobacter. 2008. PMID: 18783519 Free PMC article. Review. - Role of the Helicobacter pylori-induced inflammatory response in the development of gastric cancer.
Lamb A, Chen LF. Lamb A, et al. J Cell Biochem. 2013 Mar;114(3):491-7. doi: 10.1002/jcb.24389. J Cell Biochem. 2013. PMID: 22961880 Free PMC article. Review. - Helicobacter pylori virulence factors in gastric carcinogenesis.
Wen S, Moss SF. Wen S, et al. Cancer Lett. 2009 Sep 8;282(1):1-8. doi: 10.1016/j.canlet.2008.11.016. Epub 2008 Dec 25. Cancer Lett. 2009. PMID: 19111390 Free PMC article. Review.
Cited by
- Prevalence and Potential Risk Factors of Helicobacter pylori Infection among Asymptomatic Individuals in Kazakhstan.
Mežmale L, Polaka I, Rudzite D, Vangravs R, Kikuste I, Parshutin S, Daugule I, Tazhedinov A, Belikhina T, Igissinov N, Park JY, Herrero R, Leja M. Mežmale L, et al. Asian Pac J Cancer Prev. 2021 Feb 1;22(2):597-602. doi: 10.31557/APJCP.2021.22.2.597. Asian Pac J Cancer Prev. 2021. PMID: 33639679 Free PMC article. - Gut-brain axis interplay via STAT3 pathway: Implications of Helicobacter pylori derived secretome on inflammation and Alzheimer's disease.
Kandpal M, Baral B, Varshney N, Jain AK, Chatterji D, Meena AK, Pandey RK, Jha HC. Kandpal M, et al. Virulence. 2024 Dec;15(1):2303853. doi: 10.1080/21505594.2024.2303853. Epub 2024 Feb 4. Virulence. 2024. PMID: 38197252 Free PMC article. - In Vitro Effects of Lactobacillus plantarum LN66 and Antibiotics Used Alone or in Combination on Helicobacter pylori Mature Biofilm.
Ji J, Yang H. Ji J, et al. Microorganisms. 2021 Feb 18;9(2):424. doi: 10.3390/microorganisms9020424. Microorganisms. 2021. PMID: 33670726 Free PMC article. - Role of the Microbiota in Colorectal Cancer: Updates on Microbial Associations and Therapeutic Implications.
Coleman OI, Nunes T. Coleman OI, et al. Biores Open Access. 2016 Oct 1;5(1):279-288. doi: 10.1089/biores.2016.0028. eCollection 2016. Biores Open Access. 2016. PMID: 27790385 Free PMC article. Review. - Gastric Cancer Among American Indian and Alaska Native Populations in the United States, 2005-2016.
Melkonian SC, Pete D, Jim MA, Haverkamp D, Wiggins CL, Bruce MG, White MC. Melkonian SC, et al. Am J Gastroenterol. 2020 Dec;115(12):1989-1997. doi: 10.14309/ajg.0000000000000748. Am J Gastroenterol. 2020. PMID: 32740090 Free PMC article.
References
- Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. - PubMed
- Adachi, Y., F. Itoh, H. Yamamoto, K. Matsuno, Y. Arimura, M. Kusano, T. Endoh, Y. Hinoda, M. Oohara, M. Hosokawa, and K. Imai. 1998. Matrix metalloproteinase matrilysin (MMP-7) participates in the progression of human gastric and esophageal cancers. Int. J. Oncol. 13:1031-1035. - PubMed
- Akhiani, A. A., J. Pappo, Z. Kabok, K. Schon, W. Gao, L. E. Franzen, and N. Lycke. 2002. Protection against Helicobacter pylori infection following immunization is IL-12-dependent and mediated by Th1 cells. J. Immunol. 169:6977-6984. - PubMed
- Akhiani, A. A., K. Schon, L. E. Franzen, J. Pappo, and N. Lycke. 2004. Helicobacter pylori-specific antibodies impair the development of gastritis, facilitate bacterial colonization, and counteract resistance against infection. J. Immunol. 172:5024-5033. - PubMed
- Akhiani, A. A., A. Stensson, K. Schon, and N. Y. Lycke. 2005. IgA antibodies impair resistance against Helicobacter pylori infection: studies on immune evasion in IL-10-deficient mice. J. Immunol. 174:8144-8153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA028842/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- DK053620/DK/NIDDK NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- DK77955/DK/NIDDK NIH HHS/United States
- R01 DK058587/DK/NIDDK NIH HHS/United States
- K01 DK077955/DK/NIDDK NIH HHS/United States
- R01 DK053620/DK/NIDDK NIH HHS/United States
- P01 CA028842/CA/NCI NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- AT004821/AT/NCCIH NIH HHS/United States
- CA116087/CA/NCI NIH HHS/United States
- DK058405/DK/NIDDK NIH HHS/United States
- DK058404/DK/NIDDK NIH HHS/United States
- DK58587/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical