Cellular senescence controls fibrosis in wound healing - PubMed (original) (raw)
Cellular senescence controls fibrosis in wound healing
Joon-Il Jun et al. Aging (Albany NY). 2010 Sep.
Abstract
Mammalian wound healing involves the rapid synthesis and deposition of extracellular matrix (ECM) to maintain tissue integrity during repair. This process must be tightly controlled, as its deregulation may result in fibrosis, scarring, and loss of tissue function. Recent studies have uncovered an efficient and parsimonious mechanism for rendering fibrogenesis self-limiting in wound healing: in such diverse organs as the liver and skin, the myofibroblasts that initially proliferate and produce ECM are themselves eventually driven into senescence, blocking their further proliferation and converting them into matrix-degrading cells. Myofibroblast senescence in skin wounds is triggered by a dynamically expressed matricellular protein, CCN1/CYR61, which acts through integrin-mediated induction of oxidative stress. We propose that the onset of myofibroblast senescence is a programmed wound healing response that functions as a self-limiting mechanism for fibrogenesis, and this process may be regulated by the ECM microenvironment through the expression of CCN1/CYR61.
Conflict of interest statement
The authors of this manuscript have no conflict of interests to declare.
Figures
Figure 1.. Myofibroblast senescence imposes self-limiting control on fibrogenesis during wound healing.
Upon injury, myofibroblasts derived from activated fibroblasts and from other cell types proliferate and rapidly synthesize ECM to provide tissue integrity during repair. At later stages of wound healing, these ECM-producing myofibroblasts are themselves driven into senescence, whereupon they express an ECM-degrading phenotype characteristic of senescent cells. Therefore, fibrogenesis is self-limiting as myofibroblasts undergo senescence, thereby halting the proliferation of the ECM-producing cells and promoting ECM degradation. In cutaneous wound healing, senescence is triggered by the matricellular protein CCN1.
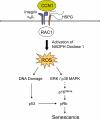
Figure 2.. A mechanistic model for CCN1-induced senescence.
The binding of CCN1 to its receptors in fibro-blasts, integrin α6β1and HSPGs, activates RAC1 and the RAC1-dependent NADPH oxidase 1 to generate a robust and sustained accumulation of ROS. This leads to a DNA damage response and activation of p53, as well as the ROS-dependent hyperactivation of ERK and p38 MAPK, leading to p16INK4a induction [22]. Both p53 and p16INK4a act upon pRb to induce senescence.
Similar articles
- CCN1/CYR61: the very model of a modern matricellular protein.
Lau LF. Lau LF. Cell Mol Life Sci. 2011 Oct;68(19):3149-63. doi: 10.1007/s00018-011-0778-3. Epub 2011 Jul 31. Cell Mol Life Sci. 2011. PMID: 21805345 Free PMC article. Review. - Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts.
Kim KH, Chen CC, Monzon RI, Lau LF. Kim KH, et al. Mol Cell Biol. 2013 May;33(10):2078-90. doi: 10.1128/MCB.00049-13. Epub 2013 Mar 18. Mol Cell Biol. 2013. PMID: 23508104 Free PMC article. - The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-β signaling.
Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, Nevzorova YA, Liedtke C, Weiskirchen R. Borkham-Kamphorst E, et al. Biochim Biophys Acta. 2014 May;1843(5):902-14. doi: 10.1016/j.bbamcr.2014.01.023. Epub 2014 Jan 31. Biochim Biophys Acta. 2014. PMID: 24487063 - The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing.
Jun JI, Lau LF. Jun JI, et al. Nat Cell Biol. 2010 Jul;12(7):676-85. doi: 10.1038/ncb2070. Epub 2010 Jun 6. Nat Cell Biol. 2010. PMID: 20526329 Free PMC article. - Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis.
Shu DY, Lovicu FJ. Shu DY, et al. Prog Retin Eye Res. 2017 Sep;60:44-65. doi: 10.1016/j.preteyeres.2017.08.001. Epub 2017 Aug 12. Prog Retin Eye Res. 2017. PMID: 28807717 Free PMC article. Review.
Cited by
- Senescent cells: a novel therapeutic target for aging and age-related diseases.
Naylor RM, Baker DJ, van Deursen JM. Naylor RM, et al. Clin Pharmacol Ther. 2013 Jan;93(1):105-16. doi: 10.1038/clpt.2012.193. Epub 2012 Dec 5. Clin Pharmacol Ther. 2013. PMID: 23212104 Free PMC article. Review. - miR-486 improves fibrotic activity in myocardial infarction by targeting SRSF3/p21-Mediated cardiac myofibroblast senescence.
Chen H, Lv L, Liang R, Guo W, Liao Z, Chen Y, Zhu K, Huang R, Zhao H, Pu Q, Yuan Z, Zeng Z, Zheng X, Feng S, Qi X, Cai D. Chen H, et al. J Cell Mol Med. 2022 Oct;26(20):5135-5149. doi: 10.1111/jcmm.17539. Epub 2022 Sep 18. J Cell Mol Med. 2022. PMID: 36117396 Free PMC article. - Sublethal heat shock induces premature senescence rather than apoptosis in human mesenchymal stem cells.
Alekseenko LL, Zemelko VI, Domnina AP, Lyublinskaya OG, Zenin VV, Pugovkina NA, Kozhukharova IV, Borodkina AV, Grinchuk TM, Fridlyanskaya II, Nikolsky NN. Alekseenko LL, et al. Cell Stress Chaperones. 2014 May;19(3):355-66. doi: 10.1007/s12192-013-0463-6. Cell Stress Chaperones. 2014. PMID: 24078383 Free PMC article. - CCN1/CYR61: the very model of a modern matricellular protein.
Lau LF. Lau LF. Cell Mol Life Sci. 2011 Oct;68(19):3149-63. doi: 10.1007/s00018-011-0778-3. Epub 2011 Jul 31. Cell Mol Life Sci. 2011. PMID: 21805345 Free PMC article. Review. - Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts.
Kim KH, Chen CC, Monzon RI, Lau LF. Kim KH, et al. Mol Cell Biol. 2013 May;33(10):2078-90. doi: 10.1128/MCB.00049-13. Epub 2013 Mar 18. Mol Cell Biol. 2013. PMID: 23508104 Free PMC article.
References
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da CM, Brown C, Popov N, Takatsu Y, Melamed J, dda di FF, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. - PubMed
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
- Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. - PubMed
- Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55:30–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL081390/HL/NHLBI NIH HHS/United States
- R01 GM078492/GM/NIGMS NIH HHS/United States
- HL081390/HL/NHLBI NIH HHS/United States
- R01 CA046565/CA/NCI NIH HHS/United States
- GM78492/GM/NIGMS NIH HHS/United States
- CA46565/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources