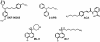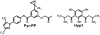Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels - PubMed (original) (raw)
Review
Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels
Christian Harteneck et al. Curr Pharm Biotechnol. 2011.
Free PMC article
Abstract
Members of the classic type of transient receptor potential channels (TRPC) represent important molecules involved in hormonal signal transduction. TRPC3/6/7 channels are of particular interest as they are components of phospholipase C driven signalling pathways. Upon receptor-activation, G-protein-mediated stimulation of phospholipase C results in breakdown of phosphatidylinositides leading to increased intracellular diacylglycerol and inositol-trisphosphate levels. Diacylglycerol activates protein kinase C, but more interestingly diacylglycerol directly activates TRPC2/3/6/7 channels. Molecular cloning, expression and characterization of TRP channels enabled reassignment of traditional inhibitors of receptor-dependent calcium entry such as SKF-96365 and 2-APB as blockers of TRPC3/6/7 and several members of non-classic TRP channels. Furthermore, several enzyme inhibitors have also been identified as TRP channel blockers, such as ACA, a phospholipase A(2) inhibitor, and W-7, a calmodulin antagonist. Finally, the naturally occurring secondary plant compound hyperforin has been identified as TRPC6-selective drug, providing an exciting proof of concept that it is possible to generate TRPC-selective channel modulators. The description of Pyr3 as the first TRPC3-selective inhibitor shows that not only nature but also man is able to generate TRP-selective modulators. The review summarizes the data on pharmacological modification of TRPC3/6/7. Sheds lights on the current knowledge and historical development of pharmacological modulators of TRPC3/6/7. Our analysis indicates that Pyr3 and hyperforin provide promising core structures for the development of new, skeletive and more potent modulators of TRPC3/6/7 activity.
Figures
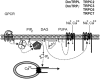
Fig. (1)
Signalling cascade leading to TRPC3/6/7 activation. Hormonal stimulation of G-protein coupled receptor (GPCR) activates phospholipase C isoforms (PLC β) via a G-protein dependent mechanism. Phospholipase C activity results in the breakdown of phosphatidylinositides (PIP2) leading to the formation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Inositol 1,4,5-trisphosphate as ligand of inositol 1,4,5-trisphosphate receptors located at the endoplasmic reticulum induce calcium release from intracellular stores, whereas DAG activates mammalian TRPC2, TRPC3, TRPC6 and TRPC7 channels. In contrast to the mammalian signalling cascade, Drosophila TRPL and TRPγ are activated by poly-unsaturated fatty acids (PUFA) generated by phospholipase A2 (PLA2) from subsequent degradation of diacylglycerols.
Fig. (2)
Chemical structures of broad range TRP channel blocker. SKF-96365: 1-{β[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl}-1H-imidazole hydrochloride; 2-APB: 2-aminoethoxydiphenyl borate; ACA: N-(p-amylcinnamoyl)anthranilic acid; ML-9: [1-(5-Chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine hydrochloride]; W-7: N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride.
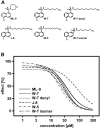
Fig. (3)
Concentration-response relationship of ML-9 and W-7 related compounds on hyperforin-induced, TRPC6-mediated fluorescence changes. A) The chemical structures of the tested compound is shown. ML-9: 1-(5-Chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine; W-7: N-(6-Aminohexyl)-5-chloro-1-naphthalenesulfonamide; W-5: N-(6-Aminohexyl)-1-naphthalenesulfonamide; W-7 isomer: N-(6-Aminohexyl)-5-chloro-2-naphthalenesulfonamide; J-8: (N-8-Aminooctyl)-5-iodo-1-naphthalenesulfonamide; W-7 decyl: N-(6-Aminodecyl)-5-chloro-1-naphthalenesulphonamide. B) Data from a representative experiment show the effect of ML-9, W-7, W-7 decyl, W-7 isomer, W-5 and J-8 on calcium entry in TRPC6-expressing cells upon hyperforin stimulation. Calcium entry was measured using FLIPRTetra and data analysed as described in Jörs et al. [5]. The data were calculated from one experiment of at least three experiments performed in quadruplicates per concentration and TRP channel. The concentration-response curves determined by calcium imaging showed a quite distinct inhibition profile. The IC50 values of ML-9, W-7, W-7 isomer, W-7 decyl W-5, J-8 were 36 µM, 26 µM, 35 µM, 29 µM, 20 µM, 85 µM, respectively.
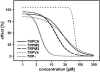
Fig. (4)
Concentration-dependent inhibition of TRPC6-, TRPM2-, TRPM3-, TRPV4- and TRPγ-mediated calcium entry by W-7. Fluorescence of TRPC6-, TRPM2-, TRPM3-, TRPV4- and TRPγ-expressing, fluo-4-loaded cells in were stimulated with hyperforin (10 µM), hydrogen peroxide (5 mM), pregnenolone sulphate (35 µM), 4α-phorbol-didecanoate (5 µM) and eicosatretranoic acid (20 µM), respectively. Calcium entry was measured using FLIPRTetra and data analysed as described in Jörs et al. [5]. The data were calculated from one experiment of at least three experiments performed in quadruplicates per concentration and TRP channel. The concentration-response curves determined by calcium imaging showed a quite distinct inhibition profile. The IC50 values to block TRPC6, TRPM2, TRPM3, TRPV4, Drosophila TRPγ were 28 µM, 26 µM, 15 µM, 65 µM, 5 µM, respectively.
Fig. (5)
Mechanistic model of the hyperforin-dependent antidepressive effect. Within the neurotransmitter circuit of release and uptake, hyperforin and classic, synthetic antidepressants attack different targets resulting in reduced presysnaptic uptake of neurotransmitter and increase concentration of neurotransmitter in the synaptic cleft. Whereas classic synthetic antidepressants directly block the neurotransmitter transporters, hyperforin activates TRPC6 channel in proximity of the neurotransmitter transporters. TRPC6 is a non-selective cation channel enabling the permeation of sodium and calcium. Sodium entry mediated by TRPC6 activation reduced the sodium gradient across the plasma membrane. As the activity of the neurotransmitter transporters depends on the electrochemical sodium gradient, TRPC6 activity indirectly inhibits neurotransmitter uptake. On the other hand, TRPC6-mediated calcium entry may trigger calcium-dependent differentiation processes responsible for changes in neuronal plasticity.
Fig. (6)
Comparison of the chemical structure of Hyp1 and Pyr-PP.
Similar articles
- Regulation of canonical transient receptor potential (TRPC) channel function by diacylglycerol and protein kinase C.
Venkatachalam K, Zheng F, Gill DL. Venkatachalam K, et al. J Biol Chem. 2003 Aug 1;278(31):29031-40. doi: 10.1074/jbc.M302751200. Epub 2003 Apr 29. J Biol Chem. 2003. PMID: 12721302 - Signalling mechanisms for TRPC3 channels.
Putney JW Jr, Trebak M, Vazquez G, Wedel B, Bird GS. Putney JW Jr, et al. Novartis Found Symp. 2004;258:123-33; discussion 133-9, 155-9, 263-6. Novartis Found Symp. 2004. PMID: 15104179 Review. - Activation of TRPC6 channels promotes endocannabinoid biosynthesis in neuronal CAD cells.
Bardell TK, Barker EL. Bardell TK, et al. Neurochem Int. 2010 Aug;57(1):76-83. doi: 10.1016/j.neuint.2010.05.002. Epub 2010 May 11. Neurochem Int. 2010. PMID: 20466028 Free PMC article. - Differences in TRPC3 and TRPC6 channels assembly in mesenteric vascular smooth muscle cells in essential hypertension.
Álvarez-Miguel I, Cidad P, Pérez-García MT, López-López JR. Álvarez-Miguel I, et al. J Physiol. 2017 Mar 1;595(5):1497-1513. doi: 10.1113/JP273327. Epub 2016 Dec 29. J Physiol. 2017. PMID: 27861908 Free PMC article. - On the potential role of source and species of diacylglycerol in phospholipase-dependent regulation of TRPC3 channels.
Vazquez G, Tano JY, Smedlund K. Vazquez G, et al. Channels (Austin). 2010 May-Jun;4(3):232-40. doi: 10.4161/chan.4.3.12058. Channels (Austin). 2010. PMID: 20458187 Review.
Cited by
- Latest Insights into the In Vivo Studies in Murine Regarding the Role of TRP Channels in Wound Healing-A Review.
Grigore A, Coman OA, Păunescu H, Costescu M, Fulga I. Grigore A, et al. Int J Mol Sci. 2024 Jun 19;25(12):6753. doi: 10.3390/ijms25126753. Int J Mol Sci. 2024. PMID: 38928459 Free PMC article. Review. - Discovery and pharmacological characterization of a novel potent inhibitor of diacylglycerol-sensitive TRPC cation channels.
Maier T, Follmann M, Hessler G, Kleemann HW, Hachtel S, Fuchs B, Weissmann N, Linz W, Schmidt T, Löhn M, Schroeter K, Wang L, Rütten H, Strübing C. Maier T, et al. Br J Pharmacol. 2015 Jul;172(14):3650-60. doi: 10.1111/bph.13151. Epub 2015 May 19. Br J Pharmacol. 2015. PMID: 25847402 Free PMC article. - Neurotensin inhibits both dopamine- and GABA-mediated inhibition of ventral tegmental area dopamine neurons.
Stuhrman K, Roseberry AG. Stuhrman K, et al. J Neurophysiol. 2015 Sep;114(3):1734-45. doi: 10.1152/jn.00279.2015. Epub 2015 Jul 15. J Neurophysiol. 2015. PMID: 26180119 Free PMC article. - Ca2+ signalling in mouse urethral smooth muscle in situ: role of Ca2+ stores and Ca2+ influx mechanisms.
Drumm BT, Rembetski BE, Cobine CA, Baker SA, Sergeant GP, Hollywood MA, Thornbury KD, Sanders KM. Drumm BT, et al. J Physiol. 2018 Apr 15;596(8):1433-1466. doi: 10.1113/JP275719. J Physiol. 2018. PMID: 29383731 Free PMC article. - Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca2+ rise in primary sensory cells.
Ding J, Xiao Y, Lu D, DU YR, Cui XY, Chen J. Ding J, et al. Neurosci Bull. 2011 Jun;27(3):135-42. doi: 10.1007/s12264-011-1018-4. Neurosci Bull. 2011. PMID: 21614096 Free PMC article.
References
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397(6716):259–263. - PubMed
- Okada T, Inoue R, Yamazaki K, Maeda A, Kurosaki T, Yamakuni T, Tanaka I, Shimizu S, Ikenaka K, Imoto K, Mori Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999;274(39):27359–27370. - PubMed
- Lucas P, Ukhanov K, Leinders-Zufall T, Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: mechanism of pheromone transduction. Neuron. 2003;40(3):551–561. - PubMed
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397(6716):255–259. - PubMed
- Jörs S, Kazanski V, Foik A, Krautwurst D, Harteneck C. Receptor-induced activation of Drosophila TRP gamma by polyunsaturated fatty acids. J. Biol. Chem. 2006;281(40):29693–29702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases