Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis - PubMed (original) (raw)
Clinical Trial
. 2010 Nov 15;70(22):9003-11.
doi: 10.1158/0008-5472.CAN-10-2364. Epub 2010 Oct 8.
Ketan R Patel, Maria Viskaduraki, James A Crowell, Marjorie Perloff, Tristan D Booth, Grygoriy Vasilinin, Ananda Sen, Anna Maria Schinas, Gianfranca Piccirilli, Karen Brown, William P Steward, Andreas J Gescher, Dean E Brenner
Affiliations
- PMID: 20935227
- PMCID: PMC2982884
- DOI: 10.1158/0008-5472.CAN-10-2364
Clinical Trial
Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis
Victoria A Brown et al. Cancer Res. 2010.
Abstract
Resveratrol, a naturally occurring polyphenol, has cancer chemopreventive properties in preclinical models. It has been shown to downregulate the levels of insulin-like growth factor-1 (IGF-I) in rodents. The purpose of the study was to assess its safety, pharmacokinetics, and effects on circulating levels of IGF-I and IGF-binding protein-3 (IGFBP-3) after repeated dosing. Forty healthy volunteers ingested resveratrol at 0.5, 1.0, 2.5, or 5.0 g daily for 29 days. Levels of resveratrol and its metabolites were measured by high performance liquid chromatography-UV in plasma obtained before and up to 24 hours after a dose between days 21 and 28. IGF-I and IGFBP-3 were measured by ELISA in plasma taken predosing and on day 29. Resveratrol was safe, but the 2.5 and 5 g doses caused mild to moderate gastrointestinal symptoms. Resveratrol-3-O-sulfate, resveratrol-4'-O-glucuronide, and resveratrol-3-O-glucuronide were major plasma metabolites. Maximal plasma levels and areas under the concentration versus time curve for the metabolites dramatically exceeded those for resveratrol, in the case of areas under the concentration versus time curve, by up to 20.3-fold. Compared with predosing values, the ingestion of resveratrol caused a decrease in circulating IGF-I and IGFBP-3 (P<0.04 for both), respectively, in all volunteers. The decrease was most marked at the 2.5 g dose level. The results suggest that repeated administration of high doses of resveratrol generates micromolar concentrations of parent and much higher levels of glucuronide and sulfate conjugates in the plasma. The observed decrease in circulating IGF-I and IGFBP-3 might contribute to cancer chemopreventive activity.
Trial registration: ClinicalTrials.gov NCT00098969.
Copyright © 2010 AACR.
Figures
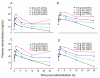
Figure 1
Mean plasma concentrations of resveratrol (A),resveratrol-4′-_O_-glucuronide (B), resveratrol-3-_O_-glucuronide (C) and resveratrol-3-_O_-sulfate (D) versus time in healthy volunteers after the last of between 21 and 28 daily doses of resveratrol at either 0.5g (black, closed circles), 1g (green, open circles), 2.5g (blue, squares) or 5g (red, triangles). Values are the mean±SD of 10 volunteers per dose level. The range of coefficients of variation (as % of the mean) for the individual data points is shown in brackets.
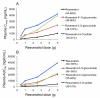
Figure 2
Relationship between dose of resveratrol and maximal plasma concentration (Cmax) (A) or area under the plasma concentration versus time curve (AUClast) (B) for resveratrol (black, rhombi), resveratrol-4′-_O_-glucuronide (red, squares), resveratrol-3-_O_-glucuronide (green, triangles) and resveratrol-3-_O_-sulfate (blue, crosses) in healthy volunteers, after a dose of resveratrol at either 0.5, 1, 2.5 or 5g ingested on between day 21 and 28 of daily dosing. Values are the mean of 10 volunteers for each dose level. The range of coefficients of variation (as % of the mean) for individual data points is shown in brackets.
Figure 3
Circulating levels of IGF-1 (A) and IGFBP-3 (B) in individual healthy volunteers before and after consumption of resveratrol at 0.5, 1.0, 2.5 or 5.0g daily for 28 days. Results of the statistical analysis by paired t-test, i.e. mean differences between pre- and post-intervention levels (pre minus post values in ng/mL), 95% confidence intervals (in brackets) and P values, are shown for each group of 10 individuals below the graphs. Negative values signify an increase rather than decrease in levels. Blood samples were taken just prior to the first dose of resveratrol and on day 29.
Figure 4
Maximal plasma concentrations (Cmax) of resveratrol (A), resveratrol-4′-_O_-glucuronide (B), resveratrol-3-_O_-glucuronide (C) and resveratrol-3-_O_-sulfate (D) in healthy volunteers who received either a single dose (open bars) or between 21 and 28 daily doses (closed bars) of resveratrol at either 0.5, 1, 2.5 or 5 g. Single dose results have been published previously (9). Values are the mean+SD of 10 volunteers at each dose level. Asterisks indicate *P<0.05,**P=0.01, ***P=0.001 and ****P<0.0005.
Similar articles
- Insulin-like growth factor-I (IGF-I) and IGF-binding proteins blood serum levels in women with early- and late-stage breast cancer: mutual relationship and possible correlations with patients' hormonal status.
Favoni RE, de Cupis A, Perrotta A, Sforzini S, Amoroso D, Pensa F, Miglietta L. Favoni RE, et al. J Cancer Res Clin Oncol. 1995;121(11):674-82. doi: 10.1007/BF01218526. J Cancer Res Clin Oncol. 1995. PMID: 7593132 - Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis.
Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, Gaunt T, Tan V, Borwick C, Emmet P, Jeffreys M, Northstone K, Rinaldi S, Thomas S, Turner SD, Pease A, Vilenchick V, Martin RM, Lewis SJ. Harrison S, et al. Cancer Causes Control. 2017 Jun;28(6):497-528. doi: 10.1007/s10552-017-0883-1. Epub 2017 Mar 30. Cancer Causes Control. 2017. PMID: 28361446 Free PMC article. - Targeted proteomics of serum IGF-I, -II, IGFBP-2, -3, -4, -5, -6 and ALS.
Albrethsen J, Drici L, Slot Vilmann LM, Holmboe SA, Thomsen CE, Rogaczewska Groendahl VL, Ottenheijm ME, Nielsen AB, Christoffersen C, Aksglaede L, Hagen CP, Wewer Albrechtsen NJ, Juul A. Albrethsen J, et al. Clin Chem Lab Med. 2025 Mar 24;63(8):1528-1537. doi: 10.1515/cclm-2024-1428. Print 2025 Jul 28. Clin Chem Lab Med. 2025. PMID: 40116411 - 24-Week jumping exercise influence on growth speed and GH-IGF-1-IGFBP-3 axis among short-stature children.
Wang H, Wang X, Wang X, Xin X, Zhang H, Jia S, Wang W. Wang H, et al. BMC Pediatr. 2025 Jul 1;25(1):476. doi: 10.1186/s12887-025-05821-3. BMC Pediatr. 2025. PMID: 40597964 Free PMC article. - Serum Insulin-Like Growth Factor Axis and the Risk of Pancreatic Cancer: Systematic Review and Meta-Analysis.
Gong Y, Zhang B, Liao Y, Tang Y, Mai C, Chen T, Tang H. Gong Y, et al. Nutrients. 2017 Apr 18;9(4):394. doi: 10.3390/nu9040394. Nutrients. 2017. PMID: 28420208 Free PMC article.
Cited by
- Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models.
Singh N, Agrawal M, Doré S. Singh N, et al. ACS Chem Neurosci. 2013 Aug 21;4(8):1151-62. doi: 10.1021/cn400094w. Epub 2013 Jun 24. ACS Chem Neurosci. 2013. PMID: 23758534 Free PMC article. Review. - Polyphenols as Possible Agents for Pancreatic Diseases.
Gašić U, Ćirić I, Pejčić T, Radenković D, Djordjević V, Radulović S, Tešić Ž. Gašić U, et al. Antioxidants (Basel). 2020 Jun 23;9(6):547. doi: 10.3390/antiox9060547. Antioxidants (Basel). 2020. PMID: 32585831 Free PMC article. Review. - Obesity-associated oxidative stress: strategies finalized to improve redox state.
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Savini I, et al. Int J Mol Sci. 2013 May 21;14(5):10497-538. doi: 10.3390/ijms140510497. Int J Mol Sci. 2013. PMID: 23698776 Free PMC article. Review. - Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases.
Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO. Reinisalo M, et al. Oxid Med Cell Longev. 2015;2015:340520. doi: 10.1155/2015/340520. Epub 2015 Jun 9. Oxid Med Cell Longev. 2015. PMID: 26180583 Free PMC article. Review. - Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review.
Gál R, Halmosi R, Gallyas F Jr, Tschida M, Mutirangura P, Tóth K, Alexy T, Czopf L. Gál R, et al. Biomedicines. 2023 Oct 25;11(11):2888. doi: 10.3390/biomedicines11112888. Biomedicines. 2023. PMID: 38001889 Free PMC article. Review.
References
- Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. - PubMed
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nature Rev Drug Discov. 2006;5:493–506. - PubMed
- Grifantini K. Understanding pathways of calorie restriction: a way to prevent cancer? J Nat Cancer Inst. 2008;100:619–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- C325/A6691/CRUK_/Cancer Research UK/United Kingdom
- NCI-N01-CN-25025/CN/NCI NIH HHS/United States
- UL1RR024986/RR/NCRR NIH HHS/United States
- N01 CN025025/CN/NCI NIH HHS/United States
- N01 CN025025/CA/NCI NIH HHS/United States
- A6894/CRUK_/Cancer Research UK/United Kingdom
- UL1 RR024986/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous