Direct dynamin-actin interactions regulate the actin cytoskeleton - PubMed (original) (raw)
Direct dynamin-actin interactions regulate the actin cytoskeleton
Changkyu Gu et al. EMBO J. 2010.
Abstract
The large GTPase dynamin assembles into higher order structures that are thought to promote endocytosis. Dynamin also regulates the actin cytoskeleton through an unknown, GTPase-dependent mechanism. Here, we identify a highly conserved site in dynamin that binds directly to actin filaments and aligns them into bundles. Point mutations in the actin-binding domain cause aberrant membrane ruffling and defective actin stress fibre formation in cells. Short actin filaments promote dynamin assembly into higher order structures, which in turn efficiently release the actin-capping protein (CP) gelsolin from barbed actin ends in vitro, allowing for elongation of actin filaments. Together, our results support a model in which assembled dynamin, generated through interactions with short actin filaments, promotes actin polymerization via displacement of actin-CPs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
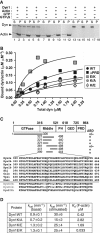
Direct dynamin–actin interactions are mediated by dynamin's middle domain. (A) Dyn1 co-sediments with F-actin. Representative Coomassie blue-stained gel of supernatants (S) and pellets (P) after centrifugation at 150 000 g in the presence and absence of 100 μM nucleotides as indicated in the figure; 1 μM dynamin was incubated with 5 μM rabbit skeletal muscle actin. (B) Actin-binding analysis of dynamin (wild-type, ΔPRD and actin-binding site mutants) to F-actin. Increasing concentrations of dyn1 were added to 2.5 μM F-actin. After centrifugation at 150 000 g, proteins were separated on SDS–PAGE and bands were analysed using densitometry. (C) Top, schematic diagram of dynamin domains and fragments tested in the actin co-sedimentation assay using IVT proteins. The presence or absence of an actin-binding domain (ABD) is indicated. Bottom, amino-acid sequence alignment of dyn2 (splice variants a and b), dyn1 (splice variants a and b), Drosophila dynamin (Shi), Caenorhabditis elegans dynamin (Cele), and yeast dynamin (Vps1). Dnm1 is a dynamin family member involved in mitochondrial morphogenesis. (D) Dynamin mutants with altered affinity for actin exhibit wild-type GTPase activities. Kinetic parameters were determined performing the GTPase assays using 0.2 μM dynamin and when indicated 80 μM lipids.
Figure 2
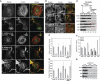
Direct dynamin–actin interactions are essential for organization of the actin cytoskeleton in podocytes. (A, B) Podocytes were first infected with adenoviruses expressing different dyn1 constructs as indicated; 18 h post-infection, cells were infected with lentivurus expressing shRNA construct S4, and dyn2 was downregulated for 3 days, after which cells were examined via immunofluorescence. Focal adhesions and F-actin were visualized with anti-paxillin antibodies and rhodamin phalloidin, respectively. In (A), white asterisk marks cells that were not infected with adenoviruses expressing dyn1 constructs. (C, D) Expression of dyn1WT, dyn1E/K, dyn1ΔPRD/EK, but not dyn1K/E or dyn1ΔPRD/KE promotes formation of stress fibres and focal adhesions in podocytes. Bar graphs depicting total F-actin (C) and the number of focal adhesions (D). Data represent measurements of >50 cells and are plotted as ±s.d. (_n_=3). (E) Effects of expression of dynamin mutants on the partitioning of actin into Triton-X 100 soluble and insoluble fractions. (Top) Flow chart of experiment, (bottom) western blot analysis of actin distribution among low-speed pellet (LSP), high-speed pellet (HSP), and high-speed supernatant (HSS) fractions in three separate experiments. (F) Bar graphs depicting the partitioning of actin in (E). The actin distribution in each fraction was expressed as a percentage of the total. Values shown are the mean ± s.d. (_n_=3) shown in (E). (G) Fractionation of α-actinin 4 by differential centrifugation in podocye lysates expressing different dynamin mutants. The result from three separate experiments are shown.
Figure 3
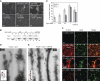
Dynamin crosslinks actin filaments into bundles. (A) Actin bundling viewed with confocal microscopy of phalloidin staining of 5 μM F-actin in control sample lacking dynamin (1), in the presence of 1 μM dyn1WT (2), 1 μM α-actinin 4 (3), 1 μM dyn1K/E (4), 1 μM dyn1E/K (5) and 1 μM dyn1WT + 200 μM GTPγS (6). Pictures were taken 1 h after initiation of the reaction. Bar is 100 μm. (B) Effects of increasing dynamin concentrations on the partitioning of actin filaments after centrifugation for 20 min at 15 000 g. Only filaments crosslinked into the bundles sediment at this speed. To generate long filaments of similar lengths, actin was polymerized in the presence of gelsolin, a barbed end-binding protein at the ratio of G1:A1000. Where indicated, 200 μM GTPγS was added. Dynamin and F-actin were detected using Coomassie blue staining of the gels. (C) Quantification of F-actin and dynamin partitioning in (B). The actin and dynamin recovery in the pellet was expressed as a percentage of the total. Values shown are mean ± s.d. (_n_=3). (D, E) Electron micrographs of actin filaments generated after 1 h in the presence of 1 μM dyn1, 5 μM Gsn–F-actin (G1:A1000), and without (D), or with 100 μM of GTPγS (E). Arrowheads indicate dynamin rings attached to actin bundles. Cartoons depict possible mechanisms by which dynamin crosslinks long actin filaments into bundles. (F) Dynamin binds and crosslinks F-actin in the presence of lipids. Actin filaments and lipid vesicles were visualized with rhodamine phalloidin (red) and fluorescein-PE (green), respectively. Reactions contained 50 μM PC:PIP2 (90:10 mol:mol), 5 μM F-actin and 0.5 μM dyn2. Single actin filaments distributed on the coverslip surface are not detected in these images because the exposure time for collecting images of the actin filament bundles was short. Bar is 100 μm, except in panel 3 where it is 20 μm.
Figure 4
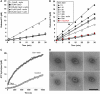
Short actin filaments promote dynamin oligomerization into ring-like structures. (A) Time course of GTP hydrolysis by 0.2 μM dyn1 incubated without or with 2.5 μM sheared F-actin treated with the indicated concentrations of Cyto D. (B) Time course of GTP hydrolysis by 0.2 μM dyn1WT or dyn1K/A (red circle) incubated with 2.5 μM Gsn or 2.5 μM Gsn-capped F-actin complexes generated with the indicated ratios of Gsn to actin. (C) A coupled assay of GTP hydrolysis by 0.2 μM dyn1 in the absence or presence of 2.5 μM Gsn-capped F-actin (G1:A100). The GTPase activity was measured at the indicated times. (D) Electron micrographs of dynamin rings formed in the presence of Gsn–F-actin (G1:A100). Bar is 100 nm. Note actin filaments around and adjacent to dynamin rings.
Figure 5
Dynamin rings dissociate gelsolin from barbed ends and promote actin elongation. (A) Solution-based actin polymerization using actin seeds. Actin seeds were generated by vortexing actin filaments for 20 s immediately before use; 0.2 μM dyn1 was added to 0.8 μM seeds or seeds capped with 5 nM CP protein. At time zero, 2 μM pyrene-labelled monomeric actin was added, and pyrene fluorescence was monitored. (B) Effects of dynamin on actin elongation; 5 μM Gsn–F-actin (G1:A1000) was incubated with 0.2 μM dyn1WT, dyn1E/K and dyn1K/A with or without 200 μM GTPγS. At time zero, 0.5 μM pyrene-labelled monomeric actin was added, and pyrene fluorescence was monitored. (C) Experiment performed as in (B) except that capped actin filaments were generated either by addition of gelsolin or CP at a 1:5 ratio to supply short actin filaments that can promote dynamin oligomerization in the absence of GTPγS. (D, E) Dynamin promotes elongation of short actin filaments capped by gelsolin. Elongation of actin filaments was measured by their ability to pellet under high-speed centrifugation. Actin was polymerized in the presence of gelsolin (G1:A10) for 20 min to generate short actin filaments that stay in the supernatant during high-speed centrifugation; 16.5 μM Gsn–F-actin complexes were incubated with 1 μM dyn1 for 30 min. Subsequently, samples were centrifuged at 150 000 g for 30 min at 22°C. Experiments were performed with dyn1WT (D) and in the presence of 5 μM recombinant GED, as indicated (E). (F) Dynamin rings displace gelsolin from the barbed ends. Effect of dynamin with or without 200 μM GTPγS on the partitioning of Gsn; 20 μM Gsn–F-actin (G1:A300) was incubated with 0.2 μM dyn1 for 30 min at RT. Samples were centrifuged at 150 000 g for 30 min. Gsn was detected using anti-Gsn antibody, dyn1 using anti-dynamin antibody and F-actin using Coomassie staining. (G) Schematic diagram of experiments performed under (H). Pyrene-labelled G-actin (3.3 μM) was polymerized for 1 h in the presence of gelsolin at the indicated ratios (G1:A200 or G1:A1000). Under these conditions, gelsolin capped >99% of the barbed ends. The Gsn–F-actin was then diluted to 0.33 μM in the presence or absence of dynamin. As G- and F-actin coexist in equilibrium, the concentration of G-actin is determined by the critical concentration (_K_d), which is defined by the on and off rates at the filament ends: 0.1 μM at the barbed (+) end, and 0.6 μM or greater at the pointed (−) end. Thus, after dilution to 0.33 μM, which lies between the critical concentrations at the two ends, Gsn–F-actin depolymerizes from the pointed ends, generating a new pool of G-actin. Depolymerization generates ∼0.23 μM pyrene G-actin that can re-polymerize in this assay, but only if the barbed ends become available. (H) Representative time courses of the re-polymerization of actin when 0.33 μM Gsn–actin complexes (G1:A200 or G1:A1000) are incubated in the presence or absence of 0.1 μM dyn1 and with or without 100 μM GTPγS. Of note, 1 μM pyrene actin represents 300–400 fluorescence units. Therefore, 75–100 units represents ∼0.25 μM F-actin, which re-polymerized at the barbed ends.
Figure 6
Dynamin–actin interactions at focal adhesions drive actin polymerization by generating free barbed ends. (A) Dynamin localizes to focal adhesions in podocytes. Podocytes were stained using anti-dynamin antibody, rhodamin phalloidin for F-actin or anti-paxillin antibody. Dynamin co-localizes with paxillin at focal adhesions and focal complexes along the membrane. (B–D) Electron micrographs of the podocyte cytoskeleton. Podocytes contain a thick actin network. Dynamin antigenic sites were visualized in the electron microscope by treatment with monoclonal anti-dynamin antibody followed by secondary antibody labelled with 10 nm gold particles (white arrow). Paxillin antigenic sites were visualized by polyclonal anti-paxillin antibody followed by secondary antibody labelled with 5 nm gold particles (black arrow). Scale bars, 200 nm. (E, F) Dynamin–actin interactions promote formation of free barbed ends in podocytes. Podocytes expressing the indicated dynamin mutants were stimulated with 5 nM EGF to induce de novo actin polymerization. After 5 min, cells were permeabilized in the presence of 0.45 μM biotin-labelled G-actin for 45 s, fixed and stained using rhodamin-conjugated anti-biotin antibody (red). In (E), total F-actin was labelled using FITC phalloidin (green). In (F), cells were stained using anti-dynamin antibody (green). Scale bars, 20 μm. (G, H) Quantification of barbed ends per cell (G) and length of the newly synthesized actin filaments (H). Data represent measurements of >20 cells (except for dyn1K/E where only 10 cells were examined) and are plotted as ±s.d. *P<0.05, **P<0.01. (I) Working model for the role of dynamin oligomerization in regulation of actin cytoskeleton. Short actin filaments are generated by gelsolin-driven cleavage of the actin filaments. Their high local concentration promotes dynamin oligomerization into rings, which in turn displace gelsolin from the barbed end and allow filament extension.
Comment in
- Actin takes its hat off to dynamin.
Roux A, Plastino J. Roux A, et al. EMBO J. 2010 Nov 3;29(21):3591-2. doi: 10.1038/emboj.2010.263. EMBO J. 2010. PMID: 21045865 Free PMC article.
Similar articles
- Dynamin regulates the dynamics and mechanical strength of the actin cytoskeleton as a multifilament actin-bundling protein.
Zhang R, Lee DM, Jimah JR, Gerassimov N, Yang C, Kim S, Luvsanjav D, Winkelman J, Mettlen M, Abrams ME, Kalia R, Keene P, Pandey P, Ravaux B, Kim JH, Ditlev JA, Zhang G, Rosen MK, Frost A, Alto NM, Gardel M, Schmid SL, Svitkina TM, Hinshaw JE, Chen EH. Zhang R, et al. Nat Cell Biol. 2020 Jun;22(6):674-688. doi: 10.1038/s41556-020-0519-7. Epub 2020 May 25. Nat Cell Biol. 2020. PMID: 32451441 Free PMC article. - Actin takes its hat off to dynamin.
Roux A, Plastino J. Roux A, et al. EMBO J. 2010 Nov 3;29(21):3591-2. doi: 10.1038/emboj.2010.263. EMBO J. 2010. PMID: 21045865 Free PMC article. - Coordinated regulation of platelet actin filament barbed ends by gelsolin and capping protein.
Barkalow K, Witke W, Kwiatkowski DJ, Hartwig JH. Barkalow K, et al. J Cell Biol. 1996 Jul;134(2):389-99. doi: 10.1083/jcb.134.2.389. J Cell Biol. 1996. PMID: 8707824 Free PMC article. - Dynamin rings: not just for fission.
Sever S, Chang J, Gu C. Sever S, et al. Traffic. 2013 Dec;14(12):1194-9. doi: 10.1111/tra.12116. Epub 2013 Sep 19. Traffic. 2013. PMID: 23980695 Free PMC article. Review. - Ever-expanding network of dynamin-interacting proteins.
Kim Y, Chang S. Kim Y, et al. Mol Neurobiol. 2006 Oct;34(2):129-36. doi: 10.1385/MN:34:2:129. Mol Neurobiol. 2006. PMID: 17220534 Review.
Cited by
- Phosphatidylserine exposure by Toxoplasma gondii is fundamental to balance the immune response granting survival of the parasite and of the host.
Santos TA, Portes Jde A, Damasceno-Sá JC, Caldas LA, Souza Wd, Damatta RA, Seabra SH. Santos TA, et al. PLoS One. 2011;6(11):e27867. doi: 10.1371/journal.pone.0027867. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140476 Free PMC article. - A dynamin-actin interaction is required for vesicle scission during endocytosis in yeast.
Palmer SE, Smaczynska-de Rooij II, Marklew CJ, Allwood EG, Mishra R, Johnson S, Goldberg MW, Ayscough KR. Palmer SE, et al. Curr Biol. 2015 Mar 30;25(7):868-78. doi: 10.1016/j.cub.2015.01.061. Epub 2015 Mar 12. Curr Biol. 2015. PMID: 25772449 Free PMC article. - Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in neuroendocrine chromaffin cells.
González-Jamett AM, Momboisse F, Guerra MJ, Ory S, Báez-Matus X, Barraza N, Calco V, Houy S, Couve E, Neely A, Martínez AD, Gasman S, Cárdenas AM. González-Jamett AM, et al. PLoS One. 2013 Aug 5;8(8):e70638. doi: 10.1371/journal.pone.0070638. Print 2013. PLoS One. 2013. PMID: 23940613 Free PMC article. - Endocytic accessory factors and regulation of clathrin-mediated endocytosis.
Merrifield CJ, Kaksonen M. Merrifield CJ, et al. Cold Spring Harb Perspect Biol. 2014 Oct 3;6(11):a016733. doi: 10.1101/cshperspect.a016733. Cold Spring Harb Perspect Biol. 2014. PMID: 25280766 Free PMC article. Review. - Insights into dynamin-associated disorders through analysis of equivalent mutations in the yeast dynamin Vps1.
Moustaq L, Smaczynska-de Rooij II, Palmer SE, Marklew CJ, Ayscough KR. Moustaq L, et al. Microb Cell. 2016 Mar 22;3(4):147-158. doi: 10.15698/mic2016.04.490. Microb Cell. 2016. PMID: 28357347 Free PMC article.
References
- Andrianantoandro E, Blanchoin L, Sept D, McCammon JA, Pollard TD (2001) Kinetic mechanism of end-to-end annealing of actin filaments. J Mol Biol 312: 721–730 - PubMed
- Arora PD, Janmey PA, McCulloch CA (1999) A role for gelsolin in stress fiber-dependent cell contraction. Exp Cell Res 250: 155–167 - PubMed
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P (2006) Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK059588/DK/NIDDK NIH HHS/United States
- R01 DK059588-10/DK/NIDDK NIH HHS/United States
- R01 DK064787/DK/NIDDK NIH HHS/United States
- R01 DK64787/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous