Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis - PubMed (original) (raw)
Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis
Gabriel Loor et al. Free Radic Biol Med. 2010.
Abstract
Low levels of reactive oxygen species (ROS) can function as redox-active signaling messengers, whereas high levels of ROS induce cellular damage. Menadione generates ROS through redox cycling, and high concentrations trigger cell death. Previous work suggests that menadione triggers cytochrome c release from mitochondria, whereas other studies implicate the activation of the mitochondrial permeability transition pore as the mediator of cell death. We investigated menadione-induced cell death in genetically modified cells lacking specific death-associated proteins. In cardiomyocytes, oxidant stress was assessed using the redox sensor RoGFP, expressed in the cytosol or the mitochondrial matrix. Menadione elicited rapid oxidation in both compartments, whereas it decreased mitochondrial potential and triggered cytochrome c redistribution to the cytosol. Cell death was attenuated by N-acetylcysteine and exogenous glutathione or by overexpression of cytosolic or mitochondria-targeted catalase. By contrast, no protection was observed in cells overexpressing Cu,Zn-SOD or Mn-SOD. Overexpression of antiapoptotic Bcl-X(L) protected against staurosporine-induced cell death, but it failed to confer protection against menadione. Genetic deletion of Bax and Bak, cytochrome c, cyclophilin D, or caspase-9 conferred no protection against menadione-induced cell death. However, cells lacking PARP-1 showed a significant decrease in menadione-induced cell death. Thus, menadione induces cell death through the generation of oxidant stress in multiple subcellular compartments, yet cytochrome c, Bax/Bak, caspase-9, and cyclophilin D are dispensable for cell death in this model. These studies suggest that multiple redundant cell death pathways are activated by menadione, but that PARP plays an essential role in mediating each of them.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
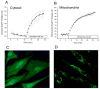
Figure 1
Oxidant stress generated by menadione in cultured cardiomyocytes. Cells expressing the redox-sensitive ratiometric sensor RoGFP were superfused with media while ratiometric images were obtained on an inverted fluorescence microscope. (A) Percent oxidation of the RoGFP sensor under baseline conditions and during menadione treatment (n=4). (B) Percent oxidation of RoGFP targeted to the mitochondrial matrix during menadione treatment (n=5). (C) Representative fluorescence image of cells expressing RoGFP. (D) Representative fluorescence image of cells expressing RoGFP targeted to the mitochondrial matrix.
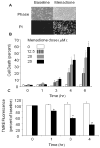
Figure 2
Menadione-induced cell death in cardiomyocytes. (A) Phase contrast images of cardiomyocytes at baseline and after 6 hrs of menadione (25 μmol/L) treatment (upper panel). Loss of plasma membrane integrity was confirmed by propidium iodide uptake (lower panels). (B) Menadione-induced cell death occurred in a time- and dose-dependent manner (Mean values ± SE, [0–3 hrs, n=4] [4–6 hrs, n=6]; * p<.05 compared with 0 hr controls). (C) Mitochondrial potential, as assessed using tetramethylrhodamine ethyl ester (TMRE). Fluorescence intensity was measured in cardiomyocytes loaded with TMRE under control conditions and during exposure to menadione (25 μmol/L). A significant decrease in TMRE fluorescence was detected as early as 1 hr after menadione treatment. Values are expressed as percent of baseline intensity (n=4; * p<.05) compared with controls. White bars indicate control cells and dark bars indicate menadione-treated cells.
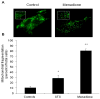
Figure 3
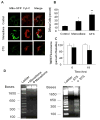
Mitochondrial morphology in cardiomyocytes treated with menadione. (A) Control cells demonstrate a normal reticular mitochondrial morphology. Cells exposed to menadione (25 μM, 4 hrs) demonstrated significant mitochondrial fragmentation. (B) Analysis of cells exhibiting fragmented mitochondrial morphology after treatment with menadione, or the apoptosis-inducing agent staurosporine (STS, 1 μM, 6 hrs). (n=3; *STS vs. controls p<0.05; **menadione vs. STS and controls p<0.05)
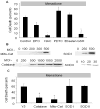
Figure 4
Effect of antioxidant therapy on menadione-induced cell death in cardiomyocytes. (A) N-acetyl-L-cysteine (NAC, 500 μmol/L) and reduced glutathione (GSH, 100 μmol/L) decreased cell death after 6 hrs of menadione (25 μmol/L) treatment compared with controls. DFO (100 μmol/L), pyrolidine dithiocarbamate (PDTC, 10 μmol/L), and ebselen (25 μmol/L) had no significant effect ([control, NAC, DFO, n=6], * p<.05 compared with control). (B) Western blot analysis of adenovirally transduced cardiomyocytes showing over-expression of catalase, mitochondria-targeted catalase, Cu, Zn-SOD and Mn-SOD after 48 hrs. (C) Cell death in adenovirally transduced cardiomyocytes treated with menadione (25 μmol/L, 6 hrs). Both cytosolic (1000 pfu/cell) and mitochondria-targeted catalase (500 pfu/cell) conferred protection compared with Y5-empty virus controls (1000 pfu/cell). Cu, Zn-SOD (SOD 1, 500 pfu/cell) and Mn-SOD over-expression (SOD 2, 20 pfu/cell) conferred no protective effect (n=3; * p<.05) compared with controls.
Figure 5
Apoptotic markers in response to menadione or staurosporine (STS). (A) Immunofluorescence analysis of cytochrome c distribution in cardiomyocytes revealed a normal mitochondrial reticular pattern that co-localized with mitochondria-targeted GFP expression under control conditions. A diffuse cytosolic staining pattern developed after menadione treatment, and after staurosporine treatment. (B) A significant increase in the percentage of cells demonstrating cytochrome c release was observed after menadione treatment or staurosporine treatment (n=10; *p<0.05 compared with controls, **p<0.05 compared with menadione). (C) TMRE fluorescence measurements of mitochondrial potential at 6 and 18 hr after STS treatment (dark bars), compared with control cells (white bars). Although a small trend was noted, this did not reach statistical significance. (D) Left: Representative gel electrophoresis showing an absence of DNA fragmentation in cells exposed to menadione (25 μM) for 6 hrs compared with controls. (lanes: 1 - ladder, 2 - control, 3 - menadione). Right: Representative gel showing the presence of DNA fragmentation after staurosporine for 6 hrs. (lanes: 1 - ladder, 2 - control, 3 - staurosporine).
Figure 6
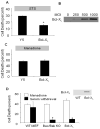
Role of mitochondrial apoptosis in staurosporine (STS)- and menadione-induced cell death in cardiomyocytes. (A) Cardiomyocytes infected with Y5 (empty vector) or Bcl-XL-expressing adenovirus (1000 MOI) for 36 hrs, then subjected to STS treatment (18 hrs) in serum-free media. LDH release into the supernatant was used to quantify cell death. Bcl-XL over-expression significantly attenuated STS-induced cell death in cardiomyocytes (n=4; * p<.05 compared with controls). (B) Bcl-XL protein over-expression levels with increasing viral titers, after 36 hr. (C) Bcl-XL over-expression (1000 MOI) in cardiomyocytes was not protective against menadione challenge compared with control cells infected with the Y5 virus (1000 MOI) (n=4). (D) Cell death in wild-type (WT) murine embryonic fibroblasts (MEFs), Bax/Bak double knockout (KO) MEFs, and Bcl-XL over-expressing MEFs in response to menadione challenge (25 μmol/L for 6 hrs) or serum withdrawal (72 hrs), as assessed by LDH release. Significant protection against serum-withdrawal-induced cell death was observed in Bax/Bak KO and Bcl-XL over-expressing cells, but these cells were not protected against menadione-induced cell death. (n=4, * p<.05)
Figure 7
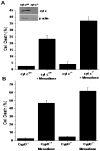
(A) Cell death (LDH Assay) in wild type (n=3) and cytochrome c−/− (n=3) murine embryonic cells in response to menadione treatment (25 μM for 6 hrs). Genetic deletion of cytochrome c did not lessen cell death in response to menadione. Inset: Immunoblot of cytochrome c protein expression. (B) Cell death (LDH Assay) in wild type (n=3) and cyclophilin-D−/− murine embryonic fibroblasts (n=3) in response to menadione treatment (25 μM for 6 hrs). Genetic deletion of Cyp-D did not lessen cell death in response to menadione.
Figure 8
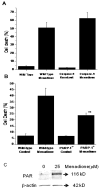
(A) Cell death (LDH Assay) in wild type (n=5) and immortalized caspase-9−/− murine embryonic fibroblasts (n=5) in response to menadione treatment (25 μM for 6 hrs). Genetic deletion of caspase-9 did not lessen cell death in response to menadione. (B) Cell death (LDH Assay) in wild type (n=4) and PARP-1−/− murine embryonic fibroblasts (n=5) in response to menadione treatment (25 μM for 6 hrs). Genetic deletion of PARP-1 significantly decreased cell death in response to menadione. (** p<.05 in comparison to wild type cells). (C) Immunoblot showing poly-(ADP-ribosylation) of cellular proteins in response to menadione (25 μM for 60 min).
Similar articles
- Mitochondrial oxidant stress triggers cell death in simulated ischemia-reperfusion.
Loor G, Kondapalli J, Iwase H, Chandel NS, Waypa GB, Guzy RD, Vanden Hoek TL, Schumacker PT. Loor G, et al. Biochim Biophys Acta. 2011 Jul;1813(7):1382-94. doi: 10.1016/j.bbamcr.2010.12.008. Epub 2010 Dec 23. Biochim Biophys Acta. 2011. PMID: 21185334 Free PMC article. - Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death.
Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Baines CP, et al. Nature. 2005 Mar 31;434(7033):658-62. doi: 10.1038/nature03434. Nature. 2005. PMID: 15800627 - Oxidative stress alters mitochondrial bioenergetics and modifies pancreatic cell death independently of cyclophilin D, resulting in an apoptosis-to-necrosis shift.
Armstrong JA, Cash NJ, Ouyang Y, Morton JC, Chvanov M, Latawiec D, Awais M, Tepikin AV, Sutton R, Criddle DN. Armstrong JA, et al. J Biol Chem. 2018 May 25;293(21):8032-8047. doi: 10.1074/jbc.RA118.003200. Epub 2018 Apr 6. J Biol Chem. 2018. PMID: 29626097 Free PMC article. - Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis?
Ying Y, Padanilam BJ. Ying Y, et al. Cell Mol Life Sci. 2016 Jun;73(11-12):2309-24. doi: 10.1007/s00018-016-2202-5. Epub 2016 Apr 5. Cell Mol Life Sci. 2016. PMID: 27048819 Free PMC article. Review. - Mitochondrial Ca2+ and Reactive Oxygen Species in Trypanosomatids.
Docampo R, Vercesi AE. Docampo R, et al. Antioxid Redox Signal. 2022 May;36(13-15):969-983. doi: 10.1089/ars.2021.0058. Epub 2021 Sep 17. Antioxid Redox Signal. 2022. PMID: 34218689 Free PMC article. Review.
Cited by
- Mitochondrial PARP1 regulates NAD+-dependent poly ADP-ribosylation of mitochondrial nucleoids.
Lee JH, Hussain M, Kim EW, Cheng SJ, Leung AKL, Fakouri NB, Croteau DL, Bohr VA. Lee JH, et al. Exp Mol Med. 2022 Dec;54(12):2135-2147. doi: 10.1038/s12276-022-00894-x. Epub 2022 Dec 6. Exp Mol Med. 2022. PMID: 36473936 Free PMC article. - Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I.
Rodríguez-Nuevo A, Torres-Sanchez A, Duran JM, De Guirior C, Martínez-Zamora MA, Böke E. Rodríguez-Nuevo A, et al. Nature. 2022 Jul;607(7920):756-761. doi: 10.1038/s41586-022-04979-5. Epub 2022 Jul 20. Nature. 2022. PMID: 35859172 Free PMC article.
References
- Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. - PubMed
- Mansfield KD, Simon MC, Keith B. Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J Appl Physiol. 2004;97:1358–1366. - PubMed
- Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. - PubMed
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxides-generating oxidase Mox1. Nature. 1999;401:79–82. - PubMed
- Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL079650-05/HL/NHLBI NIH HHS/United States
- HL35440/HL/NHLBI NIH HHS/United States
- R01 HL035440-24/HL/NHLBI NIH HHS/United States
- HL079650/HL/NHLBI NIH HHS/United States
- R21 RR025355/RR/NCRR NIH HHS/United States
- R01 HL035440/HL/NHLBI NIH HHS/United States
- R01 HL035440-23/HL/NHLBI NIH HHS/United States
- R01 HL066315/HL/NHLBI NIH HHS/United States
- R01 HL035440-22A1W1/HL/NHLBI NIH HHS/United States
- R21 RR025355-01/RR/NCRR NIH HHS/United States
- R01 HL035440-22A1/HL/NHLBI NIH HHS/United States
- R01 HL079650/HL/NHLBI NIH HHS/United States
- HL32646/HL/NHLBI NIH HHS/United States
- R01 HL066315-05/HL/NHLBI NIH HHS/United States
- R21 RR025355-02/RR/NCRR NIH HHS/United States
- R01 HL035440-15/HL/NHLBI NIH HHS/United States
- HL66315/HL/NHLBI NIH HHS/United States
- R01 HL079650-04/HL/NHLBI NIH HHS/United States
- R01 HL079650-04W1/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous