Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration - PubMed (original) (raw)
Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration
Chrysanthi Samara et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Discovery of molecular mechanisms and chemical compounds that enhance neuronal regeneration can lead to development of therapeutics to combat nervous system injuries and neurodegenerative diseases. By combining high-throughput microfluidics and femtosecond laser microsurgery, we demonstrate for the first time large-scale in vivo screens for identification of compounds that affect neurite regeneration. We performed thousands of microsurgeries at single-axon precision in the nematode Caenorhabditis elegans at a rate of 20 seconds per animal. Following surgeries, we exposed the animals to a hand-curated library of approximately one hundred small molecules and identified chemicals that significantly alter neurite regeneration. In particular, we found that the PKC kinase inhibitor staurosporine strongly modulates regeneration in a concentration- and neuronal type-specific manner. Two structurally unrelated PKC inhibitors produce similar effects. We further show that regeneration is significantly enhanced by the PKC activator prostratin.
Conflict of interest statement
Conflict of interest statement: The authors have filed patents. M.F.Y. is founder and chief scientific advisor of Entera Pharmaceuticals.
Figures
Fig. 1.
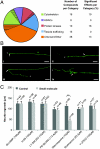
Microfluidic C. elegans manipulation for subcellular laser microsurgery and chemical library screening. (A) Micrograph of dye-filled microfluidic chip. Red, control (valve) layer; yellow, flow layer; blue, immobilization layer. Scale bar: 1 mm. (B) Animal loading from multiwell plates. The multiwell plate is held at a 40° angle and a stainless steel tube is inserted to the well bottom. (C) Microfluidic C. elegans manipulation steps. 1. Loading of nematodes. Dust, debris, air bubbles, and bacteria occasionally also enter the chip. 2. Capture of a single animal by the single aspiration channel. 3. Isolation of a single animal within the chamber by low-pressure washing of the channels to remove and recycle the rest of the nematodes. 4. Cleaning of channels by high pressure washing to remove debris and bubbles. 5. Orientation of the single animal by releasing it from the single aspiration port and recapturing it by the channel array. 6. Immobilization by pressurizing a thin membrane (see D). 7. Laser microsurgery (see E). 8. Unloading of the animal from the chip after surgery. (D) Illustration of the final immobilization process. Once a single animal is captured and linearly oriented (Left), a channel above the main chamber is pressurized pushing a thin membrane downward (Right). The membrane wraps around the animal, significantly increasing immobilization stability for imaging and surgery. Precise laser targeting of subcellular features is achieved using a femtosecond laser tightly focused inside the C. elegans body by a high numerical aperture objective lens (see Materials and Methods). (E) Software interface to accelerate axon targeting for laser axotomy. A right mouse click on the cell body is used to identify the portion of the axon at a set distance from the soma, and a left mouse click automatically moves this location to the laser focal point. (F) Average time per animal for screening steps. Total time per animal is from three independent experiments with 100 worms each.
Fig. 2.
In vivo chemical screen for small molecules affecting axonal regeneration. (A) Primary target categories of the screened compound library. The dashed parts of the pie chart represent the percentage of compounds in each category affecting regeneration. The number of screened compounds and the percentage of the effective compounds in each category are denoted. (B) Common regeneration phenotypes observed 72 h following axotomy and compound exposure: (i) no axon regrowth, (ii) forward regrowth, (iii) backward regrowth, and (iv) regrowth with branching. Arrows and asterisks indicate start and end points of regenerated axons, respectively. For regrowth with branching, indicated start and end points are for the longest regrown branch. Scale bars: 20 μm. (C) Effects of protein kinase modulators in the regeneration of PLM neurites after laser axotomy. PLM neurons of L4 nematodes were axotomized 50 μm away from the cell body. Animals were incubated in the presence of kinase modulators for 48 h, and the lengths of the longest regrowing neurites were measured (**, P ≤ 0.01). Error bars denote the SEM, n indicates the total number of animals used in each case, and each bar shows one representative screen with its controls.
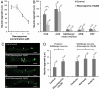
Fig. 3.
Effect of staurosporine on neurite regrowth is dependent on staurosporine concentration and neuronal type. (A) PLM neurons of L4 nematodes were axotomized, and regeneration was measured after 48 h. Staurosporine inhibited regrowth at concentrations of 5 μM or higher. Toxicity was observed at concentrations higher than 10 μM. (B) Laser microsurgeries were performed on different types of neurons in young adult nematodes, and regeneration was measured 48 h later. Staurosporine had a significant effect only in PLM neurons (**, P ≤ 0.01). (C) Regeneration phenotypes observed 48 h after axotomy of PLM (i and ii) or ALM (iii and iv) neurons in staurosporine-treated and control animals. Arrows and asterisks indicate start and end points of regenerated axons, respectively. Arrowheads in (ii) indicate terminal retraction bulb and axonal swellings formed in PLM neurons after staurosporine treatment. Scale bars: 30 μm. (D) Effect of staurosporine on GABAergic motor neurons at different parts of the nematode body. GABAergic neurons were axotomized in L4 animals and regeneration was measured after 48 h. Treatment of nematodes with 10 μM staurosporine did not significantly alter the regrowth of posterior GABAergic neurons when compared to nontreated animals. The regeneration response was similar among anterior, midbody, and posterior GABAergic neurons after exposure to staurosporine. Error bars in A, B, and D denote the SEM, and n indicates the total number of animals used.
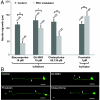
Fig. 4.
Enhancement and inhibition of regeneration by structurally different chemical modulators of PKC activity. (A) PLM neurons of L4 nematodes were axotomized, and animals were incubated for 48 h in the presence of staurosporine, Gö 6983, chelerythrine, or prostratin. The lengths of the longest regrowing neurites were compared (*, P ≤ 0.05; **, P ≤ 0.01). Error bars indicate the SEM, and n indicates the total number of animals used. (B) Representative images of regenerating PLM neurites as observed 48 h after laser microsurgery in nontreated (i), Gö 6983- (ii), chelerythrine- (iii), or prostratin-treated (iv) animals. Arrows and asterisks indicate start and end points of regenerated axons respectively. Scale bars: 30 μm.
Similar articles
- Microfluidic in vivo screen identifies compounds enhancing neuronal regeneration.
Rohde CB, Gilleland C, Samara C, Norton S, Haggarty S, Yanik MF. Rohde CB, et al. Annu Int Conf IEEE Eng Med Biol Soc. 2009;2009:5950-2. doi: 10.1109/IEMBS.2009.5334771. Annu Int Conf IEEE Eng Med Biol Soc. 2009. PMID: 19965065 - Neurosurgery: functional regeneration after laser axotomy.
Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Yanik MF, et al. Nature. 2004 Dec 16;432(7019):822. doi: 10.1038/432822a. Nature. 2004. PMID: 15602545 - Construction of a femtosecond laser microsurgery system.
Steinmeyer JD, Gilleland CL, Pardo-Martin C, Angel M, Rohde CB, Scott MA, Yanik MF. Steinmeyer JD, et al. Nat Protoc. 2010 Mar;5(3):395-407. doi: 10.1038/nprot.2010.4. Epub 2010 Feb 11. Nat Protoc. 2010. PMID: 20203659 Free PMC article. - Ultrafast laser nanosurgery in microfluidics for genome-wide screenings.
Ben-Yakar A, Bourgeois F. Ben-Yakar A, et al. Curr Opin Biotechnol. 2009 Feb;20(1):100-5. doi: 10.1016/j.copbio.2009.01.008. Epub 2009 Mar 9. Curr Opin Biotechnol. 2009. PMID: 19278850 Free PMC article. Review. - Axon regeneration mechanisms: insights from C. elegans.
Chen L, Chisholm AD. Chen L, et al. Trends Cell Biol. 2011 Oct;21(10):577-84. doi: 10.1016/j.tcb.2011.08.003. Epub 2011 Sep 8. Trends Cell Biol. 2011. PMID: 21907582 Free PMC article. Review.
Cited by
- The early bird catches the worm: new technologies for the Caenorhabditis elegans toolkit.
Xu X, Kim SK. Xu X, et al. Nat Rev Genet. 2011 Oct 4;12(11):793-801. doi: 10.1038/nrg3050. Nat Rev Genet. 2011. PMID: 21969037 Free PMC article. Review. - A fully automated microfluidic femtosecond laser axotomy platform for nerve regeneration studies in C. elegans.
Gokce SK, Guo SX, Ghorashian N, Everett WN, Jarrell T, Kottek A, Bovik AC, Ben-Yakar A. Gokce SK, et al. PLoS One. 2014 Dec 3;9(12):e113917. doi: 10.1371/journal.pone.0113917. eCollection 2014. PLoS One. 2014. PMID: 25470130 Free PMC article. - Combating neurodegenerative disease with chemical probes and model systems.
Narayan P, Ehsani S, Lindquist S. Narayan P, et al. Nat Chem Biol. 2014 Nov;10(11):911-20. doi: 10.1038/nchembio.1663. Epub 2014 Oct 17. Nat Chem Biol. 2014. PMID: 25325702 Review. - An automated microfluidic system for screening Caenorhabditis elegans behaviors using electrotaxis.
Liu D, Gupta B, Selvaganapathy PR. Liu D, et al. Biomicrofluidics. 2016 Feb 11;10(1):014117. doi: 10.1063/1.4941709. eCollection 2016 Jan. Biomicrofluidics. 2016. PMID: 26909123 Free PMC article. - High-throughput screening in the C. elegans nervous system.
Kinser HE, Pincus Z. Kinser HE, et al. Mol Cell Neurosci. 2017 Apr;80:192-197. doi: 10.1016/j.mcn.2016.06.001. Epub 2016 Jun 3. Mol Cell Neurosci. 2017. PMID: 27265309 Free PMC article. Review.
References
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. - PubMed
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. - PubMed
- Bhatt DH, Otto SJ, Depoister B, Fetcho JR. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–258. - PubMed
- Yanik MF, et al. Neurosurgery: Functional regeneration after laser axotomy. Nature. 2004;432:822. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources