Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy - PubMed (original) (raw)
. 2010 Dec;53(12):2589-99.
doi: 10.1007/s00125-010-1913-7. Epub 2010 Oct 7.
K Lemaire, G de Faudeur, N Hendrickx, M Granvik, L Van Lommel, J Mallet, G Vodjdani, P Gilon, N Binart, P in't Veld, F Schuit
Affiliations
- PMID: 20938637
- PMCID: PMC2974930
- DOI: 10.1007/s00125-010-1913-7
Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy
A Schraenen et al. Diabetologia. 2010 Dec.
Abstract
Aims/hypothesis: Upregulation of the functional beta cell mass is required to match the physiological demands of mother and fetus during pregnancy. This increase is dependent on placental lactogens (PLs) and prolactin receptors, but the mechanisms underlying these events are only partially understood. We studied the mRNA expression profile of mouse islets during pregnancy to gain a better insight into these changes.
Methods: RNA expression was measured ex vivo via microarrays and quantitative RT-PCR. In vivo observations were extended by in vitro models in which ovine PL was added to cultured mouse islets and MIN6 cells.
Results: mRNA encoding both isoforms of the rate-limiting enzyme of serotonin biosynthesis, tryptophan hydroxylase (TPH), i.e. Tph1 and Tph2, were strongly induced (fold change 25- to 200-fold) during pregnancy. This induction was mimicked by exposing islets or MIN6 cells to ovine PLs for 24 h and was dependent on janus kinase 2 and signal transducer and activator of transcription 5. Parallel to Tph1 mRNA and protein induction, islet serotonin content increased to a peak level that was 200-fold higher than basal. Interestingly, only a subpopulation of the beta cells was serotonin-positive in vitro and in vivo. The stored serotonin pool in pregnant islets and PL-treated MIN6 cells was rapidly released (turnover once every 2 h).
Conclusions/interpretation: A very strong lactogen-dependent upregulation of serotonin biosynthesis occurs in a subpopulation of mouse islet beta cells during pregnancy. Since the newly formed serotonin is rapidly released, this lactogen-induced beta cell function may serve local or endocrine tasks, the nature of which remains to be identified.
Figures
Fig. 1
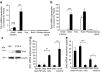
Co-expression of Tph1 and Tph2 in islets of pregnant mice. Quantitative RT-PCR analysis of mRNA encoding Tph1 (a) and Tph2 (b) in islets and in tissue as indicated from non-pregnant (white bars) and P15.5 females (black bars). Results are mean ± SEM of three to seven independent experiments (see also ESM Fig. 1). c Representative western blot of TPH1 protein levels in islets isolated from non-pregnant (NP) and P15.5 mice. β-Actin was used as control for protein load. Microarray analysis (Affymetrix MOE430_2.0) of mRNA encoding Tph1 (d) and Tph2 (e) in islets of non-pregnant (NP) female mice, FACS-purified beta cells (male mice) and other tissues as indicated. Primary beta cells expressed more Tph2 and less Tph1 than the MIN6 cell line. Brain and small intestine were used as controls. *p < 0.05, **p < 0.01 and ***p < 0.001 for difference between tested conditions
Fig. 2
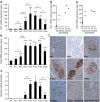
Time course of islet Tph1 and Tph2 mRNA expression and serotonin content during pregnancy. Expression of Tph1 (a) and Tph2 mRNA (b) in islets isolated from non-pregnant (NP) mice, pregnant mice on different days of pregnancy as indicated and mice at 10 days postpartum (10 dpp). Expression was determined with the MoGene_1.0_ST array. Values are mean ± SD, n = 3–4. Tph1 mRNA expression peaked around P12.5 to P15.5, whereas that of Tph2 plateaued from P9.5 onwards. Expression of both declined after delivery. See ESM Fig. 2a for Tph1 expression in intestine at different time points during pregnancy. c Time course of serotonin content in islets isolated from mice as indicated (n = 2–11). For the content of different parts of the intestine, see ESM Fig. 3. a–c *p < 0.05, **p < 0.01 and ***p < 0.001 for difference between non-pregnant and pregnant, or between indicated conditions. d Correlation analysis between islet Tph1 or (e) Tph2 mRNA expression and serotonin content. The correlation between Tph1 (r = 0.951) and serotonin was better than that between Tph2 (r = 0.812) and serotonin. f Serotonin immunostaining (brown) of pancreatic sections from pregnant mice. Serotonin was used as blocking agent. Only a subset of the islet cells was stained with an intensity similar to the time-dependent ELISA measurement of serotonin content of isolated islets (c). No immunostaining was detected in _Tph1_-KO mice
Fig. 3
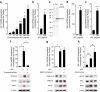
Placental lactogens induce Tph1 and Tph2 expression in cultured beta cells. Tph1 mRNA (a) was induced in MIN6 cells by ovine PL (oPL) in a concentration-dependent manner. Effect, in MIN6 cells, of 500 ng/ml oPL (24 h) on Tph2 mRNA (b) and TPH1 protein (c); the immunoblot in c is representative of three independent experiments. Quantitative RT-PCR data are mean ± SEM, n = 3. Effect of 24 h 500 ng/ml oPL on Tph1 mRNA (d) and Tph2 mRNA (e) in islets cultured as monolayers after isolation from non-pregnant mice. MIN6 cells incubated with oPL were treated with specific inhibitors of three signalling pathways, namely tyrphostin AG490 (50 μmol/l) for JAK2–STAT5 (f), MEK1/2-inhibitor-1 (10 μmol/l) for RAS–RAF–MAPK (g) and wortmannin (100 nmol/l) for PI3K (h). Tph1 expression was quantified via quantitative RT-PCR (mean ± SEM, n = 3–4) and TPH1 protein via western blotting (blots are representative of three to four experiments). The JAK2 inhibitor tyrphostin AG490 reduced Tph1 expression in MIN6 cells treated with oPL. For expression of Prlr mRNA in islets during pregnancy, see ESM Fig. 4. *p < 0.05, **p < 0.01 and ***p < 0.001 for difference between non-treated controls and treated conditions, as analysed by Student’s t test (MIN6 cells) and Z test (islets cultured in monolayers)
Fig. 4
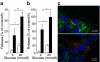
Dissociated release of serotonin and insulin from pregnant islets and PL-stimulated MIN6 cells. a Insulin (white bars) and serotonin (black bars) release, expressed as per cent of total content per h from pancreatic islets freshly isolated from P15.5 females, showed a different pattern (for details on content, see ESM Fig. 5c). Release and content were measured after 1 h incubation in 5 or 20 mmol/l glucose. b Similar results were obtained for insulin and serotonin release of ovine PL-treated MIN6 cells incubated for 1 h in 2 or 20 mmol/l glucose (for details on content, see ESM Fig. 5d). The change in insulin content during pregnancy is shown in ESM Fig. 5a; insulin release from islets isolated from non-pregnant vs pregnant mice is shown in ESM Fig. 5b. Values (a, b) are mean ± SEM; n = 4. *p < 0.05 and **p < 0.01 for differences between indicated conditions. c Non-overlapping serotonin and insulin immunostaining in the same MIN6-cell. Two representative microscopic images of MIN6 cells treated for 24 h with 500 ng/ml ovine PL after double immunostaining for serotonin (red) and insulin (green). Cell nuclei were stained with Hoechst (blue)
Fig. 5
A subpopulation of PL-stimulated beta cells was induced to produce serotonin. a Confocal microscopy of MIN6 cells treated for 24 h with 500 ng/ml ovine PL (oPL). Double immunostaining for TPH1 (green) and serotonin (red). Nuclei were stained with Hoechst (blue). Immunoreactive TPH1 and serotonin are present in the same cell. b Presence of serotonin (red) in a subset of PL-stimulated insulin-containing MIN6 cells (insulin, green). c Per cent of serotonin-positive MIN6 cells after treatment with different concentrations of oPL for 24 h; values mean ± SEM, n = 3 except for 100 ng/ml where n = 2; counting done blind by three independent persons. *p < 0.05 and ***p < 0.001 for difference between non-treated controls and treated conditions. d Confocal microscopy of islets treated for 24 h with 500 ng/ml oPL, showing overlays of immunoreactivity for TPH1 (green) and serotonin (red), insulin (green) and serotonin (red), and glucagon (green) and serotonin (red). Data indicate that a subpopulation of primary islet beta cells contains serotonin after stimulation with PL
Similar articles
- Expression mechanism of tryptophan hydroxylase 1 in mouse islets during pregnancy.
Iida H, Ogihara T, Min MK, Hara A, Kim YG, Fujimaki K, Tamaki M, Fujitani Y, Kim H, Watada H. Iida H, et al. J Mol Endocrinol. 2015 Aug;55(1):41-53. doi: 10.1530/JME-14-0299. Epub 2015 Jul 1. J Mol Endocrinol. 2015. PMID: 26136513 - Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes.
Goyvaerts L, Lemaire K, Arijs I, Auffret J, Granvik M, Van Lommel L, Binart N, in't Veld P, Schuit F, Schraenen A. Goyvaerts L, et al. PLoS One. 2015 Mar 27;10(3):e0121868. doi: 10.1371/journal.pone.0121868. eCollection 2015. PLoS One. 2015. PMID: 25816302 Free PMC article. - Serotonin regulates pancreatic beta cell mass during pregnancy.
Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, Yang K, Honig G, van der Hart M, Kishimoto N, Wang J, Yagihashi S, Tecott LH, Watada H, German MS. Kim H, et al. Nat Med. 2010 Jul;16(7):804-8. doi: 10.1038/nm.2173. Epub 2010 Jun 27. Nat Med. 2010. PMID: 20581837 Free PMC article. - Serotonin competence of mouse beta cells during pregnancy.
Goyvaerts L, Schraenen A, Schuit F. Goyvaerts L, et al. Diabetologia. 2016 Jul;59(7):1356-1363. doi: 10.1007/s00125-016-3951-2. Epub 2016 Apr 7. Diabetologia. 2016. PMID: 27056372 Review. - β-Cell adaptation in pregnancy.
Baeyens L, Hindi S, Sorenson RL, German MS. Baeyens L, et al. Diabetes Obes Metab. 2016 Sep;18 Suppl 1(Suppl 1):63-70. doi: 10.1111/dom.12716. Diabetes Obes Metab. 2016. PMID: 27615133 Free PMC article. Review.
Cited by
- Research resource: RNA-Seq reveals unique features of the pancreatic β-cell transcriptome.
Ku GM, Kim H, Vaughn IW, Hangauer MJ, Myung Oh C, German MS, McManus MT. Ku GM, et al. Mol Endocrinol. 2012 Oct;26(10):1783-92. doi: 10.1210/me.2012-1176. Epub 2012 Aug 21. Mol Endocrinol. 2012. PMID: 22915829 Free PMC article. - Mouse Models of Gestational Diabetes Mellitus and Its Subtypes: Recent Insights and Pitfalls.
Grupe K, Scherneck S. Grupe K, et al. Int J Mol Sci. 2023 Mar 22;24(6):5982. doi: 10.3390/ijms24065982. Int J Mol Sci. 2023. PMID: 36983056 Free PMC article. Review. - Serotonin transporter protects the placental cells against apoptosis in caspase 3-independent pathway.
Hadden C, Fahmi T, Cooper A, Savenka AV, Lupashin VV, Roberts DJ, Maroteaux L, Hauguel-de Mouzon S, Kilic F. Hadden C, et al. J Cell Physiol. 2017 Dec;232(12):3520-3529. doi: 10.1002/jcp.25812. Epub 2017 Apr 12. J Cell Physiol. 2017. PMID: 28109119 Free PMC article. - Advances in tryptophan hydroxylase-2 gene expression regulation: new insights into serotonin-stress interaction and clinical implications.
Chen GL, Miller GM. Chen GL, et al. Am J Med Genet B Neuropsychiatr Genet. 2012 Mar;159B(2):152-71. doi: 10.1002/ajmg.b.32023. Am J Med Genet B Neuropsychiatr Genet. 2012. PMID: 22241550 Free PMC article. Review. - Augmented Stat5 Signaling Bypasses Multiple Impediments to Lactogen-Mediated Proliferation in Human β-Cells.
Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, Wang P, Bender AS, Frank SJ, Stewart AF. Chen H, et al. Diabetes. 2015 Nov;64(11):3784-97. doi: 10.2337/db15-0083. Epub 2015 Jul 9. Diabetes. 2015. PMID: 26159175 Free PMC article.
References
- Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr. 2000;54(Suppl 1):S47–S51. - PubMed
- Brelje TC, Svensson AM, Stout LE, Bhagroo NV, Sorenson RL. An immunohistochemical approach to monitor the prolactin-induced activation of the JAK2/STAT5 pathway in pancreatic islets of Langerhans. J Histochem Cytochem. 2002;50:365–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases