Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials - PubMed (original) (raw)
Review
Reboxetine for acute treatment of major depression: systematic review and meta-analysis of published and unpublished placebo and selective serotonin reuptake inhibitor controlled trials
Dirk Eyding et al. BMJ. 2010.
Abstract
Objectives: To assess the benefits and harms of reboxetine versus placebo or selective serotonin reuptake inhibitors (SSRIs) in the acute treatment of depression, and to measure the impact of potential publication bias in trials of reboxetine.
Design: Systematic review and meta-analysis including unpublished data.
Data sources: Bibliographic databases (Medline, Embase, PsycINFO, BIOSIS, and Cochrane Library), clinical trial registries, trial results databases, and regulatory authority websites up until February 2009, as well as unpublished data from the manufacturer of reboxetine (Pfizer, Berlin).
Eligibility criteria: Double blind, randomised, controlled trials of acute treatment (six weeks or more) with reboxetine versus placebo or SSRIs in adults with major depression.
Outcome measures: Remission and response rates (benefit outcomes), as well as rates of patients with at least one adverse event and withdrawals owing to adverse events (harm outcomes).
Data extraction and data synthesis: The procedures for data extraction and assessment of risk of bias were always conducted by one person and checked by another. If feasible, data were pooled by meta-analyses (random effects model). Publication bias was measured by comparing results of published and unpublished trials.
Results: We analysed 13 acute treatment trials that were placebo controlled, SSRI controlled, or both, which included 4098 patients. Data on 74% (3033/4098) of these patients were unpublished. In the reboxetine versus placebo comparison, no significant differences in remission rates were shown (odds ratio 1.17, 95% confidence interval 0.91 to 1.51; P=0.216). Substantial heterogeneity (I(2)=67.3%) was shown in the meta-analysis of the eight trials that investigated response rates for reboxetine versus placebo. A sensitivity analysis that excluded a small inpatient trial showed no significant difference in response rates between patients receiving reboxetine and those receiving placebo (OR 1.24, 95% CI 0.98 to 1.56; P=0.071; I(2)=42.1%). Reboxetine was inferior to SSRIs (fluoxetine, paroxetine, and citalopram) for remission rates (OR 0.80, 95% CI 0.67 to 0.96; P=0.015) and response rates (OR 0.80, 95% CI 0.67 to 0.95; P=0.01). Reboxetine was inferior to placebo for both harm outcomes (P<0.001 for both), and to fluoxetine for withdrawals owing to adverse events (OR 1.79, 95% CI 1.06 to 3.05; P=0.031). Published data overestimated the benefit of reboxetine versus placebo by up to 115% and reboxetine versus SSRIs by up to 23%, and also underestimated harm.
Conclusions: Reboxetine is, overall, an ineffective and potentially harmful antidepressant. Published evidence is affected by publication bias, underlining the urgent need for mandatory publication of trial data.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi\_disclosure.pdf (available on request from the corresponding author) and declare: no support from any company for the submitted work; DE was employed by H Lundbeck A/S, Copenhagen, between January 2006 and April 2007; MH received remuneration from Boehringer Ingelheim and Lilly Pharma for three talks on depression guidelines in 2008; and UG, MK, TK, MFK, and BW are employees of IQWiG. DE is a former employee of IQWiG. In order to produce unbiased health technology assessment reports, the institute depends on access to all of the relevant data on the topic under investigation. The authors therefore support the mandatory worldwide establishment of trial registries and study results databases. ML and MH were involved in the development of the German Disease Management Guideline on Depression.
Figures
Fig 1 Flowchart of study selection. *Excluding long term acute treatment trial
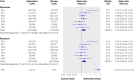
Fig 2 Forest plot showing meta-analyses of remission and response rates for trials that compared reboxetine with placebo. Empty boxes show published studies and filled boxes show unpublished studies. Study 091 is not included in the pooled analysis of response of reboxetine versus placebo because of high heterogeneity (see text for details). CI, confidence interval; n, number of patients with event; N, number of patients in treatment group
Fig 3 Forest plot showing meta-analyses of remission and response rates for trials that compared reboxetine with selective serotonin reuptake inhibitors (SSRIs; fluoxetine, paroxetine, and citalopram). Empty boxes show published studies and filled boxes show unpublished studies. Empty diamonds show subtotals (individual SSRIs) and filled diamonds show overall totals (all SSRIs). CI, confidence interval; n, number of patients with event; N, number of patients in treatment group
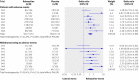
Fig 4 Forest plot showing meta-analyses of rates of patients with at least one adverse event and rates of withdrawals owing to adverse events for trials that compared reboxetine with placebo. Empty boxes show published studies and filled boxes show unpublished studies. CI, confidence interval; n, number of patients with event; N, number of patients in treatment group
Fig 5 Forest plot showing meta-analyses of rates of patients with at least one adverse event and rates of withdrawals owing to adverse events for trials that compared reboxetine with selective serotonin reuptake inhibitors (SSRIs; fluoxetine and paroxetine). Empty boxes show published studies and filled boxes show unpublished studies. Empty diamonds show subtotals (individual SSRIs) and filled diamonds show overall totals (all SSRIs). CI, confidence interval; n, number of patients with event; N, number of patients in treatment group
Fig 6 Forest plot showing meta-analyses of published, unpublished, and all trials. Publication bias (right column) is presented as the ratio of odds ratios of published results versus overall results. The extent of publication bias is expressed as percentage change between the analysis of published trials only and the analysis of all trials (that is, publication bias=100×(ORpublished data/ORtotal data–1)). *Fluoxetine controlled studies only
Comment in
- Missing clinical trial data: setting the record straight.
Godlee F, Loder E. Godlee F, et al. BMJ. 2010 Oct 12;341:c5641. doi: 10.1136/bmj.c5641. BMJ. 2010. PMID: 20940217 No abstract available. - Reboxetine in depression. NICE guidance differs, so where next?
Krishnadas R. Krishnadas R. BMJ. 2010 Nov 16;341:c6484. doi: 10.1136/bmj.c6484. BMJ. 2010. PMID: 21081613 No abstract available. - Reboxetine in depression. All the relevant data?
Turner EH. Turner EH. BMJ. 2010 Nov 16;341:c6487. doi: 10.1136/bmj.c6487. BMJ. 2010. PMID: 21081614 No abstract available. - Review: reboxetine is ineffective and potentially harmful for acute treatment of major depression.
Katzman MA, Tsirgielis D. Katzman MA, et al. Evid Based Ment Health. 2011 May;14(2):48. doi: 10.1136/ebmh.14.2.48. Evid Based Ment Health. 2011. PMID: 21502154 No abstract available.
Similar articles
- Agomelatine versus other antidepressive agents for major depression.
Guaiana G, Gupta S, Chiodo D, Davies SJ, Haederle K, Koesters M. Guaiana G, et al. Cochrane Database Syst Rev. 2013 Dec 17;2013(12):CD008851. doi: 10.1002/14651858.CD008851.pub2. Cochrane Database Syst Rev. 2013. PMID: 24343836 Free PMC article. Review. - Paroxetine versus other anti-depressive agents for depression.
Purgato M, Papola D, Gastaldon C, Trespidi C, Magni LR, Rizzo C, Furukawa TA, Watanabe N, Cipriani A, Barbui C. Purgato M, et al. Cochrane Database Syst Rev. 2014 Apr 3;2014(4):CD006531. doi: 10.1002/14651858.CD006531.pub2. Cochrane Database Syst Rev. 2014. PMID: 24696195 Free PMC article. Review. - Citalopram versus other anti-depressive agents for depression.
Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, Churchill R, Watanabe N, Barbui C. Cipriani A, et al. Cochrane Database Syst Rev. 2012 Jul 11;2012(7):CD006534. doi: 10.1002/14651858.CD006534.pub2. Cochrane Database Syst Rev. 2012. PMID: 22786497 Free PMC article. Review. - Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery.
Mead GE, Hsieh CF, Lee R, Kutlubaev MA, Claxton A, Hankey GJ, Hackett ML. Mead GE, et al. Cochrane Database Syst Rev. 2012 Nov 14;11(11):CD009286. doi: 10.1002/14651858.CD009286.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152272 Free PMC article. Updated. Review. - Newer generation antidepressants for depressive disorders in children and adolescents.
Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN. Hetrick SE, et al. Cochrane Database Syst Rev. 2012 Nov 14;11(11):CD004851. doi: 10.1002/14651858.CD004851.pub3. Cochrane Database Syst Rev. 2012. PMID: 23152227 Free PMC article. Review.
Cited by
- [Effects of COVID-19 on the consumption of antidepressants in the Province of Santa Cruz de Tenerife, Canary Islands].
Sapino I, Oliva Martín A, Dévora Gutiérrez S, Abdala Kuri S. Sapino I, et al. Farm Comunitarios. 2022 Dec 18;15(1):64-71. doi: 10.33620/FC.2173-9218.(2023).07. eCollection 2023 Jan 2. Farm Comunitarios. 2022. PMID: 39156192 Free PMC article. Spanish. - Sensitivity analysis with iterative outlier detection for systematic reviews and meta-analyses.
Meng Z, Wang J, Lin L, Wu C. Meng Z, et al. Stat Med. 2024 Apr 15;43(8):1549-1563. doi: 10.1002/sim.10008. Epub 2024 Feb 6. Stat Med. 2024. PMID: 38318993 - Trends in the Consumption of Antidepressant Drugs before and during the COVID-19 Pandemic in the Canary Islands, Spain: The Case of the Province of Las Palmas.
Moreno V, Dévora S, Abdala-Kuri S, Oliva A. Moreno V, et al. Healthcare (Basel). 2023 May 15;11(10):1425. doi: 10.3390/healthcare11101425. Healthcare (Basel). 2023. PMID: 37239712 Free PMC article. - Clinical study reports published by the European Medicines Agency 2016-2018: a cross-sectional analysis.
Byrne D, Prendergast C, Fahey T, Moriarty F. Byrne D, et al. BMJ Open. 2023 May 15;13(5):e068981. doi: 10.1136/bmjopen-2022-068981. BMJ Open. 2023. PMID: 37188475 Free PMC article. - A pharmacological challenge paradigm to assess neural signatures of script-elicited acute dissociation in women with post-traumatic stress disorder.
Mertens YL, Manthey A, Sierk A, de Jong P, Walter H, Daniels JK. Mertens YL, et al. BJPsych Open. 2023 May 2;9(3):e78. doi: 10.1192/bjo.2023.34. BJPsych Open. 2023. PMID: 37128866 Free PMC article.
References
- Pharmacia Limited. Summary of product characteristics: Edronax 4 mg tablets. 2009. www.medicines.org.uk/EMC/medicine/8386/SPC/Edronax+4mg+Tablets/#INDICATIONS<.>
- Preskorn SH. Reboxetine: a norepinephrine selective reuptake pump inhibitor. J Psychiatr Pract 2004;10:57-63. - PubMed
- Schwabe U, Paffrath D. [Drug prescription report] [German]. Springer, 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical