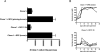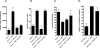CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes - PubMed (original) (raw)
CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes
Rinke Bos et al. Cancer Res. 2010.
Abstract
CD4 help for CD8(+) T lymphocytes prevents tolerance and promotes the survival of effector and memory CD8(+) T cells. Here, we describe additional helper functions that require CD4(+) T cells within the tumor environment. CD8(+) T-cell recruitment, proliferation, and effector function within the tumor were greatly enhanced by tumor-specific CD4(+) T cells. Recruitment of CD8(+) T cells was accelerated by IFN-γ-dependent production of chemokines. Production of interleukin-2 by tumor resident CD4(+) T cells enhanced CD8(+) T-cell proliferation and upregulated expression of granzyme B. These results highlight a novel role for tumor-specific CD4(+) T cells in promoting CD8(+) T-cell recruitment and cytolytic function, two previously unappreciated aspects of tumor-specific CD4 help.
©2010 AACR.
Figures
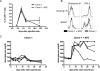
Figure 1. Anti-tumor efficacy of Clone-1 CD8+ T cells
RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE cells were injected i.v. A. On the indicated days PBL were analyzed by FACS for Thy1.1+ cells. B. Effector function of Clone-1 cells was measured in the spleen and draining lymph node of the vaccination site at day 6 by intra-cellular staining of Granzyme B and IFN-γ. C. Glucose levels in the blood were measured at the indicated time points and each line represents one mouse. Data are representative of 2 independent experiments with 4 mice per group.
Figure 2. CD4+ T cells are required in the tumor environment, for accumulation of Clone-1 cells and anti-tumor immunity
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE or DO11.10 cells were injected i.v. One group of mice received HA-Kd peptide and 200 μg poly(I:C) in IFA s.c. in the left flank and SFE peptide and 200 μg poly(I:C) in IFA s.c. in the right flank. Pancreata were analyzed by FACS at day 6. Data are cumulative over 4 experiments. B. Glucose levels in the blood were measured at the indicated time points and each line represents one mouse. Data are representative of 2 independent experiments with 4 mice per group.
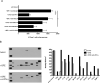
Figure 3. Tumor-specific CD4+ T cells induce an inflammatory environment that promotes recruitment of Clone-1 cells
A. RIP-Tag2-HA mice were immunized with or without injection of 0.3×106 SFE cells. At day 6, 1×106 in vitro activated Clone-1 cells were injected and recruitment of Clone-1 cells to the pancreas was analyzed 40 hours later. The indicated neutralizing antibodies were injected at day 6 and 7. Data are cumulative over 3 experiments. **, P<0.005. B. RIP-Tag2-HA mice were immunized with or without injection of SFE cells (0.3×106) 6 days prior to isolation of pancreata and one group was injected with IFN-γ neutralizing antibodies at day 4 and 5. 200 μg of cell lysate was used for a cytokine array. Array images are from 30 minute exposures to X-ray film and quantitated data show relative changes between conditions. Data are representative of 2 independent experiments.
Figure 4. CD4+ T cells in the tumor environment stimulate proliferation and survival
RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE or DO11.10 cells were injected i.v. Tumor infiltrating Clone-1 cells were analyzed at day 6 by FACS for proliferation (A: Ki-67) and Bim expression (B). The gate of the Ki-67 staining was based on the Thy1.1− Ki-67− cells in the pancreas. Control stainings in the histograms show staining with the Bim antibody of Bim−/− splenocytes. Dot plots and histograms are representative examples of each condition and bar graphs depict cumulative data of 3 experiments with 2–4 mice per group.
Figure 5. The role of IL-2 production by CD4+ T cells
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE, 2×106 IL-2−/− SFE or 2×106 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies at day 4 and 5. Pancreata were analyzed by FACS at day 6. Data are cumulative over 2 experiments with 7 mice per group. B. Mice were injected as described in A. Expression of markers for proliferation (B: Ki-67) and survival (C: Bim) on Clone-1 cells were analyzed by FACS at day 6. Data are cumulative over 3 experiments with 9 mice per group. D. RIP-Tag2-HA mice were immunized with or without i.v. injection of 0.3×106 SFE or 2×106 SFE IL-2−/−. At day 6, 1×106 in vitro activated Clone-1 cells were injected and recruitment of Clone-1 cells to the pancreas was analyzed 4 days later. Data are cumulative over 3 experiments with 9 mice per group.
Figure 6. IL-2 production by CD4+ T cells is critical for increased effector function and anti-tumor efficacy of Clone-1 cells
A. RIP-Tag2-HA mice were immunized and Clone-1 cells (0.3×106) with or without 0.3×106 SFE, 0.3×106 DO11.10, 2×106 IL-2−/− SFE or 2×106 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies at day 4 and 5. Pancreata were isolated at day 6. Data are cumulative over 3 experiments with 9 mice per group. B. RIP-Tag2-HA mice were immunized and Clone-1 cells (1×105) with or without 1×105 SFE, 7×105 IL-2−/− SFE or 7×105 SFE cells were injected i.v. The latter group was injected with IFN-γ neutralizing antibodies daily starting at day 4 through 13 followed by injections every 2 days. Glucose levels in the blood were measured at the indicated time points. Data are representative of 2 independent experiments with 3–5 mice per group. Each line represents one mouse.
Similar articles
- Antibody-independent antiviral function of memory CD4+ T cells in vivo requires regulatory signals from CD8+ effector T cells.
Zhong W, Roberts AD, Woodland DL. Zhong W, et al. J Immunol. 2001 Aug 1;167(3):1379-86. doi: 10.4049/jimmunol.167.3.1379. J Immunol. 2001. PMID: 11466356 - Intratumoral interleukin-21 increases antitumor immunity, tumor-infiltrating CD8+ T-cell density and activity, and enlarges draining lymph nodes.
Søndergaard H, Galsgaard ED, Bartholomaeussen M, Straten PT, Odum N, Skak K. Søndergaard H, et al. J Immunother. 2010 Apr;33(3):236-49. doi: 10.1097/CJI.0b013e3181c0c1cb. J Immunother. 2010. PMID: 20445344 - Altered T-Cell Balance in Lymphoid Organs of a Mouse Model of Colorectal Cancer.
Tanner SM, Daft JG, Hill SA, Martin CA, Lorenz RG. Tanner SM, et al. J Histochem Cytochem. 2016 Dec;64(12):753-767. doi: 10.1369/0022155416672418. Epub 2016 Oct 20. J Histochem Cytochem. 2016. PMID: 27798287 Free PMC article. - Adoptive transfer of tumor-primed, in vitro-activated, CD4+ T effector cells (TEs) combined with CD8+ TEs provides intratumoral TE proliferation and synergistic antitumor response.
Wang LX, Shu S, Disis ML, Plautz GE. Wang LX, et al. Blood. 2007 Jun 1;109(11):4865-76. doi: 10.1182/blood-2006-09-045245. Epub 2007 Feb 6. Blood. 2007. PMID: 17284532 Free PMC article. - Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy.
Hu HM, Winter H, Urba WJ, Fox BA. Hu HM, et al. J Immunol. 2000 Oct 15;165(8):4246-53. doi: 10.4049/jimmunol.165.8.4246. J Immunol. 2000. PMID: 11035058
Cited by
- Enhanced anti-tumor efficacy of electroporation (EP)-mediated DNA vaccine boosted by allogeneic lymphocytes in pre-established tumor models.
Shi S, Zhang L, Zheng A, Xie F, Kesse S, Yang Y, Peng J, Xu Y. Shi S, et al. Cancer Immunol Immunother. 2024 Oct 3;73(12):248. doi: 10.1007/s00262-024-03838-8. Cancer Immunol Immunother. 2024. PMID: 39358555 Free PMC article. - Prognostic value of tumor-infiltrating lymphocytes and PD-L1 expression in esophageal squamous cell carcinoma.
Hu J, Toyozumi T, Murakami K, Endo S, Matsumoto Y, Otsuka R, Shiraishi T, Iida S, Morishita H, Makiyama T, Nishioka Y, Uesato M, Hayano K, Nakano A, Matsubara H. Hu J, et al. Cancer Med. 2024 Sep;13(17):e70179. doi: 10.1002/cam4.70179. Cancer Med. 2024. PMID: 39264227 Free PMC article. - MDM2 inhibitors in cancer immunotherapy: Current status and perspective.
Zeng Q, Zeng S, Dai X, Ding Y, Huang C, Ruan R, Xiong J, Tang X, Deng J. Zeng Q, et al. Genes Dis. 2024 Mar 28;11(6):101279. doi: 10.1016/j.gendis.2024.101279. eCollection 2024 Nov. Genes Dis. 2024. PMID: 39263534 Free PMC article. Review. - Sculpting the tumour microenvironment by combining radiotherapy and ATR inhibition for curative-intent adjuvant immunotherapy.
Patin EC, Nenclares P, Chan Wah Hak C, Dillon MT, Patrikeev A, McLaughlin M, Grove L, Foo S, Soliman H, Barata JP, Marsden J, Baldock H, Gkantalis J, Roulstone V, Kyula J, Burley A, Hubbard L, Pedersen M, Smith SA, Clancy-Thompson E, Melcher AA, Ono M, Rullan A, Harrington KJ. Patin EC, et al. Nat Commun. 2024 Aug 13;15(1):6923. doi: 10.1038/s41467-024-51236-6. Nat Commun. 2024. PMID: 39134540 Free PMC article. - Trim21-mediated CCT2 ubiquitination suppresses malignant progression and promotes CD4+T cell activation in breast cancer.
Chen X, Ma C, Li Y, Liang Y, Chen T, Han D, Luo D, Zhang N, Zhao W, Wang L, Chen B, Guo H, Yang Q. Chen X, et al. Cell Death Dis. 2024 Jul 30;15(7):542. doi: 10.1038/s41419-024-06944-8. Cell Death Dis. 2024. PMID: 39079960 Free PMC article.
References
- Peterson AC, Harlin H, Gajewski TF. Immunization with Melan-A peptide-pulsed peripheral blood mononuclear cells plus recombinant human interleukin-12 induces clinical activity and T-cell responses in advanced melanoma. J Clin Oncol. 2003;21:2342–8. - PubMed
- Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. - PubMed
- Mocellin S, Mandruzzato S, Bronte V, Lise M, Nitti D. Part I: Vaccines for solid tumours. Lancet Oncol. 2004;5:681–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials