ARID1A mutations in endometriosis-associated ovarian carcinomas - PubMed (original) (raw)
. 2010 Oct 14;363(16):1532-43.
doi: 10.1056/NEJMoa1008433. Epub 2010 Sep 8.
Sohrab P Shah, Osama M Al-Agha, Yongjun Zhao, Kane Tse, Thomas Zeng, Janine Senz, Melissa K McConechy, Michael S Anglesio, Steve E Kalloger, Winnie Yang, Alireza Heravi-Moussavi, Ryan Giuliany, Christine Chow, John Fee, Abdalnasser Zayed, Leah Prentice, Nataliya Melnyk, Gulisa Turashvili, Allen D Delaney, Jason Madore, Stephen Yip, Andrew W McPherson, Gavin Ha, Lynda Bell, Sian Fereday, Angela Tam, Laura Galletta, Patricia N Tonin, Diane Provencher, Dianne Miller, Steven J M Jones, Richard A Moore, Gregg B Morin, Arusha Oloumi, Niki Boyd, Samuel A Aparicio, Ie-Ming Shih, Anne-Marie Mes-Masson, David D Bowtell, Martin Hirst, Blake Gilks, Marco A Marra, David G Huntsman
Affiliations
- PMID: 20942669
- PMCID: PMC2976679
- DOI: 10.1056/NEJMoa1008433
ARID1A mutations in endometriosis-associated ovarian carcinomas
Kimberly C Wiegand et al. N Engl J Med. 2010.
Abstract
Background: Ovarian clear-cell and endometrioid carcinomas may arise from endometriosis, but the molecular events involved in this transformation have not been described.
Methods: We sequenced the whole transcriptomes of 18 ovarian clear-cell carcinomas and 1 ovarian clear-cell carcinoma cell line and found somatic mutations in ARID1A (the AT-rich interactive domain 1A [SWI-like] gene) in 6 of the samples. ARID1A encodes BAF250a, a key component of the SWI–SNF chromatin remodeling complex. We sequenced ARID1A in an additional 210 ovarian carcinomas and a second ovarian clear-cell carcinoma cell line and measured BAF250a expression by means of immunohistochemical analysis in an additional 455 ovarian carcinomas.
Results: ARID1A mutations were seen in 55 of 119 ovarian clear-cell carcinomas (46%), 10 of 33 endometrioid carcinomas (30%), and none of the 76 high-grade serous ovarian carcinomas. Seventeen carcinomas had two somatic mutations each. Loss of the BAF250a protein correlated strongly with the ovarian clear-cell carcinoma and endometrioid carcinoma subtypes and the presence of ARID1A mutations. In two patients, ARID1A mutations and loss of BAF250a expression were evident in the tumor and contiguous atypical endometriosis but not in distant endometriotic lesions.
Conclusions: These data implicate ARID1A as a tumor-suppressor gene frequently disrupted in ovarian clear-cell and endometrioid carcinomas. Since ARID1A mutation and loss of BAF250a can be seen in the preneoplastic lesions, we speculate that this is an early event in the transformation of endometriosis into cancer. (Funded by the British Columbia Cancer Foundation and the Vancouver General Hospital–University of British Columbia Hospital Foundation.).
Figures
Figure 1. Mutations Found in ARID1A and the BAF250a Protein It Encodes
The 20 exons of ARID1A are represented (as numbered gray boxes) above a schematic of the BAF250a protein (the blue segment, with the ARID [AT-rich interactive domain] DNA-binding domain in pink, the HIC1 [hypermethylated in cancer 1] binding domain in green, and the three C-terminal leucine-rich LXXLL motifs that facilitate interaction with gluco-corticoid receptor in yellow). The nucleotide mutations (with corresponding amino acid mutations in parentheses) listed above the schematic are those identified by means of transcriptome sequencing (RNA sequencing) of the 18 samples of ovarian clear-cell carcinoma and the TOV21G cell line in the discovery cohort, and those listed below the schematic were identified in subsequent validation efforts with the use of targeted exon resequencing and Sanger sequencing of genomic DNA from the 210 ovarian-cancer samples in the mutation-validation cohort. All unique somatic mutations detected in samples of ovarian clear-cell carcinoma, endometrioid carcinoma, and high-grade serous carcinoma are shown. Numbers 1 through 6858 below the schematic indicate the nucleotide (nt) position, starting with the A in the ATG start codon for ARID1A in position 1 (based on the sequence given in record number NM_006015.4 in Entrez Gene; also see Table 1 in the Supplementary Appendix). UTR denotes untranslated region.
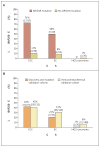
Figure 2. Results of Immunohistochemical Analyses of BAF250a Expression
The percentages of tumors (with number and total number in parentheses) from three subtypes of ovarian cancer — clear-cell carcinoma (CCC), endometrioid carcinoma (EC), and high-grade serous (HGS) carcinoma — from the discovery and mutation-validation cohorts that showed loss of BAF250a expression are shown in Panel A for samples with and samples without ARID1A mutations and in Panel B for samples in the discovery and mutation-validation cohorts and samples in the immunohistochemical validation cohort. The rate of BAF250a loss was higher among CCC specimens with an ARID1A mutation than among those without an ARID1A mutation (P<0.001); the same was true for EC specimens (P = 0.02). The loss of expression was also consistently more common in CCC and EC (the two endometriosis-associated carcinomas) than in HGS carcinoma when assessed in the discovery and mutation-validation cohorts and again in the immunohistochemical validation cohort (Panel B), with P<0.001 for all comparisons. All P values were calculated with the use of Fisher’s exact test.
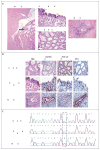
Figure 3. Analysis of Ovarian Clear-Cell arcinoma and Associated Endometriosis in a Study Patient
Panel A shows a section (hematoxylin and eosin [H&E]) on which a clear-cell carcinoma (black arrow) has arisen in an endometriotic cyst (white arrow). The same section, viewed at a higher magnification, shows regions of the clear-cell carcinoma and contiguous atypical endometriosis. A region of distant endometriosis from the same patient is also shown. Panel B shows the results of immunohistochemical staining of the epithelial portions of tissue specimens shown in Panel A for expression of BAF250a, hepatocyte nuclear factor 1β (HNF-1β), and estrogen receptor (ER). BAF250a immunoreactivity is lost in both the clear-cell carcinoma and the contiguous atypical endometriosis but is maintained in the distant endometriosis. Both regions of endometriosis differ from the carcinoma in their lack of HNF-1β expression (with weak expression in the contiguous atypical endometriosis) and maintenance of estrogen-receptor expression. Panel C shows sequencing chromatograms for the clear-cell carcinoma and polymerase-chain-reaction (PCR) clones of microdissected material from the contiguous atypical endometriosis and distant endometriosis, from which DNA was extracted. The carcinoma and contiguous atypical endometriosis show nucleotide variation corresponding to G6139T (as indicated with the dashed box); the tumor shows a heterozygous peak at that location, whereas the atypical endometriosis is homozygous for the substitution (in 17 of 42 clones). In contrast, the distant endometriosis shows wild-type sequence (in all 52 clones analyzed). None of the PCR clones from the distant endometriosis showed variation from the wild-type sequence.
Comment in
- The origin of ovarian cancer—is it getting clearer?
Birrer MJ. Birrer MJ. N Engl J Med. 2010 Oct 14;363(16):1574-5. doi: 10.1056/NEJMe1009527. N Engl J Med. 2010. PMID: 20942676 No abstract available. - Endometriosis-associated ovarian carcinomas.
Birnbaum DJ, Birnbaum D, Bertucci F. Birnbaum DJ, et al. N Engl J Med. 2011 Feb 3;364(5):483-4; author reply 484-5. doi: 10.1056/NEJMc1012780. N Engl J Med. 2011. PMID: 21288104 No abstract available. - Endometriosis-associated ovarian carcinomas.
Nissenblatt M. Nissenblatt M. N Engl J Med. 2011 Feb 3;364(5):482-3; author reply 484-5. doi: 10.1056/NEJMc1012780. N Engl J Med. 2011. PMID: 21288105 No abstract available.
Similar articles
- A functional proteogenomic analysis of endometrioid and clear cell carcinomas using reverse phase protein array and mutation analysis: protein expression is histotype-specific and loss of ARID1A/BAF250a is associated with AKT phosphorylation.
Wiegand KC, Hennessy BT, Leung S, Wang Y, Ju Z, McGahren M, Kalloger SE, Finlayson S, Stemke-Hale K, Lu Y, Zhang F, Anglesio MS, Gilks B, Mills GB, Huntsman DG, Carey MS. Wiegand KC, et al. BMC Cancer. 2014 Feb 22;14:120. doi: 10.1186/1471-2407-14-120. BMC Cancer. 2014. PMID: 24559118 Free PMC article. - Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma.
Ayhan A, Mao TL, Seckin T, Wu CH, Guan B, Ogawa H, Futagami M, Mizukami H, Yokoyama Y, Kurman RJ, Shih IeM. Ayhan A, et al. Int J Gynecol Cancer. 2012 Oct;22(8):1310-5. doi: 10.1097/IGC.0b013e31826b5dcc. Int J Gynecol Cancer. 2012. PMID: 22976498 Free PMC article. - Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers.
Lowery WJ, Schildkraut JM, Akushevich L, Bentley R, Marks JR, Huntsman D, Berchuck A. Lowery WJ, et al. Int J Gynecol Cancer. 2012 Jan;22(1):9-14. doi: 10.1097/IGC.0b013e318231f140. Int J Gynecol Cancer. 2012. PMID: 22193641 Free PMC article. - Common genetic changes between endometriosis and ovarian cancer.
Obata K, Hoshiai H. Obata K, et al. Gynecol Obstet Invest. 2000;50 Suppl 1:39-43. doi: 10.1159/000052877. Gynecol Obstet Invest. 2000. PMID: 11093060 Review. - ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas.
Samartzis EP, Noske A, Dedes KJ, Fink D, Imesch P. Samartzis EP, et al. Int J Mol Sci. 2013 Sep 12;14(9):18824-49. doi: 10.3390/ijms140918824. Int J Mol Sci. 2013. PMID: 24036443 Free PMC article. Review.
Cited by
- Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer.
Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, Maitra A, Pollack JR. Shain AH, et al. Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):E252-9. doi: 10.1073/pnas.1114817109. Epub 2012 Jan 10. Proc Natl Acad Sci U S A. 2012. PMID: 22233809 Free PMC article. - Frontiers in Bladder Cancer Genomic Research.
Li Y, Sun L, Guo X, Mo N, Zhang J, Li C. Li Y, et al. Front Oncol. 2021 May 20;11:670729. doi: 10.3389/fonc.2021.670729. eCollection 2021. Front Oncol. 2021. PMID: 34094968 Free PMC article. Review. - ARID1A-dependent maintenance of H3.3 is required for repressive CHD4-ZMYND8 chromatin interactions at super-enhancers.
Reske JJ, Wilson MR, Armistead B, Harkins S, Perez C, Hrit J, Adams M, Rothbart SB, Missmer SA, Fazleabas AT, Chandler RL. Reske JJ, et al. BMC Biol. 2022 Sep 25;20(1):209. doi: 10.1186/s12915-022-01407-y. BMC Biol. 2022. PMID: 36153585 Free PMC article. - Modulation of estrogenic action in clear cell carcinoma of the ovary (Review).
Tanase Y, Yamada Y, Shigetomi H, Kajihara H, Oonogi A, Yoshizawa Y, Furukawa N, Haruta S, Yoshida S, Sado T, Oi H, Kobayashi H. Tanase Y, et al. Exp Ther Med. 2012 Jan;3(1):18-24. doi: 10.3892/etm.2011.376. Epub 2011 Oct 24. Exp Ther Med. 2012. PMID: 22969838 Free PMC article. - Genomic Landscape of Endometrial, Ovarian, and Cervical Cancers in Japan from the Database in the Center for Cancer Genomics and Advanced Therapeutics.
Xi Q, Kage H, Ogawa M, Matsunaga A, Nishijima A, Sone K, Kawana K, Oda K. Xi Q, et al. Cancers (Basel). 2023 Dec 27;16(1):136. doi: 10.3390/cancers16010136. Cancers (Basel). 2023. PMID: 38201563 Free PMC article.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010 July 7; (Epub ahead of print) - PubMed
- Köbel M, Kalloger SE, Huntsman DG, et al. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol. 2010;29:203–11. - PubMed
- Tavassoli FA, Devilee P, editors. World Health Organization of classification of tumours. Vol. 4. Lyon, France: IARC Press; 2003. Pathology and genetics of tumours of the breast and female genital organs.
Publication types
MeSH terms
Substances
Grants and funding
- STP-53912/CAPMC/ CIHR/Canada
- R01CA129080/CA/NCI NIH HHS/United States
- R01 CA103937/CA/NCI NIH HHS/United States
- R01 CA103937-07/CA/NCI NIH HHS/United States
- R01 CA129080/CA/NCI NIH HHS/United States
- R01 CA129080-04/CA/NCI NIH HHS/United States
- R01CA103937/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases