Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain - PubMed (original) (raw)
Rubicon and PLEKHM1 negatively regulate the endocytic/autophagic pathway via a novel Rab7-binding domain
Keisuke Tabata et al. Mol Biol Cell. 2010 Dec.
Abstract
The endocytic and autophagic pathways are involved in the membrane trafficking of exogenous and endogenous materials to lysosomes. However, the mechanisms that regulate these pathways are largely unknown. We previously reported that Rubicon, a Beclin 1-binding protein, negatively regulates both the autophagic and endocytic pathways by unidentified mechanisms. In this study, we performed database searches to identify potential Rubicon homologues that share the common C-terminal domain, termed the RH domain. One of them, PLEKHM1, the causative gene of osteopetrosis, also suppresses endocytic transport but not autophagosome maturation. Rubicon and PLEKHM1 specifically and directly interact with Rab7 via their RH domain, and this interaction is critical for their function. Furthermore, we show that Rubicon but not PLEKHM1 uniquely regulates membrane trafficking via simultaneously binding both Rab7 and PI3-kinase.
Figures
Figure 1.
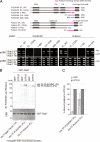
PLEKHM1 and Rubicon preferentially bind directly to the GTP-bound form of Rab7. (A) Deletion analysis of PLEKHM1 and Rubicon. Deletion series of PLEKHM1 and Rubicon in which the indicated deletions in the Rubicon homology (RH) domains and CGHL mutagenesis are shown with magenta and asterisks (****), respectively. The interactions between the PLEKHM1 or Rubicon deletion mutants and Rab7 were examined by a yeast two-hybrid assay. In all cases, two independent colonies were tested. (B and C) The nucleotide dependency was examined by an in vitro GST pulldown assay as described in Materials and Methods. (C) Recombinant PLEKHM1 and Rubicon proteins were detected with anti-PLEKHM1 and anti-Rubicon antibodies. GST-Rab7 was visualized by staining with Coomassie Brilliant Blue (CBB). (D) The relative binding amounts were quantified and are shown as percentages.
Figure 2.
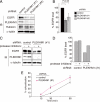
The effects of PLEKHM1 overexpression on the endocytic pathway. (A and B) (A) EGFR degradation was impaired by overexpressing PLEKHM1. A representative anti-EGFR immunoblot of EGF-stimulated A549 cells. The same membrane was immunoblotted with an anti-α-tubulin antibody as a loading control. (B) The immunodetectable levels of EGFR (relative to α-tubulin) remained constant in PKLEMH1 cells relative to the controls (mean ± SEM). (C) EGF transport was disrupted by overexpressing PLEKHM1. EGF transport to lysosomes was evaluated based on the colocalization of EGF and dextran. (D) The percentages of colocalization are shown as merged EGF/total EGF (mean ± SD, n > 20). Bars, 20 μm. (E) The effects of PLEKHM1 overexpression on EGF uptake. The EGF intensities 15 min after adding EGF are shown as percentages (mean ± SD, n > 20). (F) Accumulation of EGF on early endosomes in PLEKHM1-overexpressing cells. Cells were stained with an anti-EEA1 antibody, an early endosomal marker, and an anti-LAMP1 antibody, a late endosomal/lysosomal marker. Bars, 20 μm.
Figure 3.
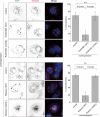
The effects of PLEKHM1 knockdown on the endocytic pathway. (A and B) The effects of PLEKHM1 knockdown on the EGFR levels. (A) Whole cell extracts from knockdown cells were analyzed by immunoblotting using the indicated antibodies. The experiments were performed in triplicate and a representative gel is shown. (B) Each protein level was normalized to the α-tubulin level and expressed as a percentage relative to control. Data are shown as the mean ± SEM. (C and D) PLEKHM1 knockdown enhances the degradation of EGFR. (C) Knockdown cells were repeatedly treated with lysosomal inhibitors (1 mg/ml leupeptin and 5 μg/ml E-64d) for 50 h and subjected to immunoblotting. (D) The EGFR levels were normalized to the α-tubulin levels and are shown as a percentage relative to the control. (E) The effects of knockdown on the efficiency of EGF transport to LAMP1-positive endosomes. Knocked down cells were stained with an anti-LAMP1 antibody at each time point. The percentages of colocalization are shown as merged EGF/total EGF (mean ± SD, n > 20). Colocalization images are shown in Supplementary Figure S4.
Figure 4.
The localization of PLEKHM1 and Rubicon is dependent on the Rab7 interaction. (A) The effects of coexpressing Rab7 mutants on the localization of mStrawberry-PLEKHM1 and mStrawberry-Rubicon. HeLa cells were transiently cotransfected with the indicated plasmids and then examined by confocal microscopy. Bars, 20 μm. (B) Mutants that cannot interact with the GTP-bound form of Rab7, such as PLEKHM1 (FL_CGHL), PLEKHM1 (1–895), and Rubicon (FL_CGHL), localize to the cytoplasm. HeLa cells were transfected with each GFP-tagged mutant. Bars, 20 μm.
Figure 5.
The interactions with Rab7 are important for the regulation of the endocytic pathway. The effects of overexpressing the non–Rab7-binding CGHL mutant on endocytic transport were evaluated by assessing the transport of EGF to lysosomes as shown in Figure 2, C and D. The percentages of colocalization are shown as merged EGF/total EGF (mean ± SD, n > 30). Bars, 20 μm.
Figure 6.
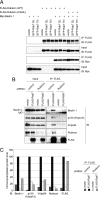
Rubicon mediates simultaneous binding between the Beclin 1–PI3-kinase complex and Rab7. (A) The interaction between Beclin 1 and Rubicon is Rab7-independent. Lysates from HEK293A cells expressing a Rab7 mutant, Myc-Beclin 1 and FLAG-Rubicon (wild-type) or FLAG-Rubicon (CGHL) mutant were immunoprecipitated with anti-FLAG M2 beads. (B and C) Rubicon mediates simultaneous binding between the endogenous Beclin 1–PI3-kinase complex and Rab7. (B) Lysates from knocked down cells that were transiently transfected with the vector control or FLAG-Rab7 were immunoprecipitated with anti-FLAG M2 beads and subjected to immunoblotting with the indicated antibodies. (C) The immunoprecipitated protein levels in B were quantified.
Figure 7.
The Beclin 1–associating (BA) domain has a dominant-negative effect on Beclin 1 binding. Schematic view of the mouse Rubicon (mRubicon) structure and its deletion mutants. The mRubicon deletion mutants were subjected to immunoprecipitation analyses in HEK293A cells to map the Beclin 1–binding region. An anti-RFP antibody was used to detect mStrawberry (mSt) and mCherry. Lysates from HEK293A cells expressing each plasmid were immunoprecipitated with an anti-Beclin 1 antibody and immunoblotted with an anti-Rubicon antibody. An asterisk indicates a nonspecific band.
Figure 8.
The ability of Rubicon to interact with both Rab7 and Beclin 1 is important to regulate the endocytic pathway. (A and B) The effects of overexpressing the BA domain (393–521) on the simultaneous interactions among Beclin 1, Rubicon, and Rab7. (A) Coimmunoprecipitation of endogenous Beclin 1 with Rab7 was disrupted in HEK293A cells overexpressing the BA domain, whereas Rubicon coimmunoprecipitated with Rab7 in these cells. An asterisk indicates a nonspecific band. (B) Immunoprecipitated protein levels in A were quantified. (C and D) The effects of overexpressing the BA domain on EGFR degradation. (C) EGFR levels were monitored in A549 cells overexpressing the BA domain as shown in Figure 2A. (D) Immunodetectable levels of EGFR (relative to α-tubulin) remained constant in cells overexpressing the BA domain relative to the controls (mean ± SEM). An anti-RFP antibody was used to detect mCherry-mRubicon (393–521). (E) PLEKHM1 and Rubicon negatively regulate the endocytic pathway through their interactions with Rab7. Their interactions with Rab7 are also required for their localization to endosomal membranes. Rubicon mediates the simultaneous interaction between the Beclin 1–PI3-kinase complex and Rab7, which is an important step in the regulation of the endocytic pathway and autophagosome maturation.
Similar articles
- PLEKHM1 regulates autophagosome-lysosome fusion through HOPS complex and LC3/GABARAP proteins.
McEwan DG, Popovic D, Gubas A, Terawaki S, Suzuki H, Stadel D, Coxon FP, Miranda de Stegmann D, Bhogaraju S, Maddi K, Kirchof A, Gatti E, Helfrich MH, Wakatsuki S, Behrends C, Pierre P, Dikic I. McEwan DG, et al. Mol Cell. 2015 Jan 8;57(1):39-54. doi: 10.1016/j.molcel.2014.11.006. Epub 2014 Dec 11. Mol Cell. 2015. PMID: 25498145 - The Rab7 effector PLEKHM1 binds Arl8b to promote cargo traffic to lysosomes.
Marwaha R, Arya SB, Jagga D, Kaur H, Tuli A, Sharma M. Marwaha R, et al. J Cell Biol. 2017 Apr 3;216(4):1051-1070. doi: 10.1083/jcb.201607085. Epub 2017 Mar 21. J Cell Biol. 2017. PMID: 28325809 Free PMC article. - PLEKHM1: Adapting to life at the lysosome.
McEwan DG, Dikic I. McEwan DG, et al. Autophagy. 2015 Apr 3;11(4):720-2. doi: 10.1080/15548627.2015.1034419. Autophagy. 2015. PMID: 25905573 Free PMC article. - Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.
Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Salminen A, et al. Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review. - Overview of macroautophagy regulation in mammalian cells.
Mehrpour M, Esclatine A, Beau I, Codogno P. Mehrpour M, et al. Cell Res. 2010 Jul;20(7):748-62. doi: 10.1038/cr.2010.82. Epub 2010 Jun 15. Cell Res. 2010. PMID: 20548331 Review.
Cited by
- Loss of RUBCN/rubicon in adipocytes mediates the upregulation of autophagy to promote the fasting response.
Yamamuro T, Nakamura S, Yanagawa K, Tokumura A, Kawabata T, Fukuhara A, Teranishi H, Hamasaki M, Shimomura I, Yoshimori T. Yamamuro T, et al. Autophagy. 2022 Nov;18(11):2686-2696. doi: 10.1080/15548627.2022.2047341. Epub 2022 Mar 14. Autophagy. 2022. PMID: 35282767 Free PMC article. - RUN and FYVE domain-containing protein 4 enhances autophagy and lysosome tethering in response to Interleukin-4.
Terawaki S, Camosseto V, Prete F, Wenger T, Papadopoulos A, Rondeau C, Combes A, Rodriguez Rodrigues C, Vu Manh TP, Fallet M, English L, Santamaria R, Soares AR, Weil T, Hammad H, Desjardins M, Gorvel JP, Santos MA, Gatti E, Pierre P. Terawaki S, et al. J Cell Biol. 2015 Sep 28;210(7):1133-52. doi: 10.1083/jcb.201501059. J Cell Biol. 2015. PMID: 26416964 Free PMC article. - Atg8 family LC3/GABARAP proteins are crucial for autophagosome-lysosome fusion but not autophagosome formation during PINK1/Parkin mitophagy and starvation.
Nguyen TN, Padman BS, Usher J, Oorschot V, Ramm G, Lazarou M. Nguyen TN, et al. J Cell Biol. 2016 Dec 19;215(6):857-874. doi: 10.1083/jcb.201607039. Epub 2016 Nov 18. J Cell Biol. 2016. PMID: 27864321 Free PMC article. - Molecular Mechanism of Autophagosome-Lysosome Fusion in Mammalian Cells.
Ke PY. Ke PY. Cells. 2024 Mar 13;13(6):500. doi: 10.3390/cells13060500. Cells. 2024. PMID: 38534345 Free PMC article. Review. - Osteopetrosis: genetics, treatment and new insights into osteoclast function.
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Sobacchi C, et al. Nat Rev Endocrinol. 2013 Sep;9(9):522-36. doi: 10.1038/nrendo.2013.137. Epub 2013 Jul 23. Nat Rev Endocrinol. 2013. PMID: 23877423 Review.
References
- Burda P., Padilla S. M., Sarkar S., Emr S. D. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J. Cell Sci. 2002;115:3889–3900. - PubMed
- Cao C., Laporte J., Backer J. M., Wandinger-Ness A., Stein M. P. Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic. 2007;8:1052–1067. - PubMed
- Carlton J., Bujny M., Peter B. J., Oorschot V.M.J., Rutherford A., Mellor H., Klumperman J., McMahon H. T., Cullen P. J. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr. Biol. 2004;14:1791–1800. - PubMed
- Choi J. H., Hong W. P., Kim M. J., Kim J. H., Ryu S. H., Suh P. G. Sorting nexin 16 regulates EGF receptor trafficking by phosphatidylinositol-3-phosphate interaction with the Phox domain. J. Cell Sci. 2004;117:4209–4218. - PubMed
- Christoforidis S., Zerial M. Purification and identification of novel Rab effectors using affinity chromatography. Methods. 2000;20:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials