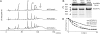Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus - PubMed (original) (raw)
Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus
Magda L Atilano et al. Proc Natl Acad Sci U S A. 2010.
Abstract
The cell wall of Staphylococcus aureus is characterized by an extremely high degree of cross-linking within its peptidoglycan (PGN). Penicillin-binding protein 4 (PBP4) is required for the synthesis of this highly cross-linked peptidoglycan. We found that wall teichoic acids, glycopolymers attached to the peptidoglycan and important for virulence in Gram-positive bacteria, act as temporal and spatial regulators of PGN metabolism, controlling the level of cross-linking by regulating PBP4 localization. PBP4 normally localizes at the division septum, but in the absence of wall teichoic acids synthesis, it becomes dispersed throughout the entire cell membrane and is unable to function normally. As a consequence, the peptidoglycan of TagO null mutants, impaired in wall teichoic acid biosynthesis, has a decreased degree of cross-linking, which renders it more susceptible to the action of lysozyme, an enzyme produced by different host organisms as an initial defense against bacterial infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Highly cross-linked muropeptides, resulting from PBP4 activity, are less abundant in the peptidoglycan of a S. aureus ΔtagO mutant. (A) HPLC analysis of mutanolysin-digested PGN of the parental strain NCTC8325-4 and mutants NCTCΔ_tagO_ and NCTCΔ_pbpD_. Arrow points to highly cross-linked muropeptide species, which are less abundant in the mutants lacking TagO and PBP4. (I–V) Muropeptide species from monomers to pentamers. (B) Western blot analysis, using a specific anti-PBP4 antibody, of membrane proteins from the same three strains showing that PBP4 is expressed at wild-type levels in the NCTCΔ_tagO_ background. (C) Analysis of membrane proteins labeled with Bocillin-FL and separated by SDS/PAGE, showing that PBP4 is able to bind Bocillin-FL in the NCTCΔ_tagO_ background. (D) Lysozyme hydrolysis of purified PGN from NCTC8325-4, NCTCΔ_tagO_, and NCTCΔ_pbpD_, followed by monitoring the decrease in absorbance at OD600nm, and showing that PGN with a lower degree of cross-linking had an increased susceptibility to lysozyme.
Fig. 2.
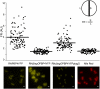
Septal localization of PBP4 is lost in a tagO null mutant. Microscopy images and quantification of septum versus lateral membrane fluorescence (fluorescence ratio, FR) of PBP4–YFP in a wild-type background (RNPBP4YFP), a Δ_tagO_ background (RNΔ_tagO_PBP4YFP), and a Δ_tagO_ mutant complemented with plasmid-encoded tagO (RNΔ_tagO_PBP4YFPp_tagO_). Also shown are RNPBP4YFP cells labeled with membrane dye Nile Red, which is homogeneously distributed in the cell membrane. Quantification was performed in 100 cells displaying closed septa for each strain. Horizontal lines correspond to average FR values. FR values over 2 indicate preferential septal localization whereas FR values equal to or under 2 indicate that a protein is dispersed over the cell surface. p values < 10−7. Scale bar: 1 μm.
Fig. 3.
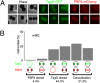
TagO protein is recruited to the division septum before PBP4. (A) The strain RNTagOPBP4, expressing simultaneously TagO–GFP and PBP4–mCherry protein fusions, was analyzed by fluorescence microscopy. (Top) Results showing that TagO reaches the division septum before PBP4 in several cells. (Bottom) Cells in which PBP4 and TagO colocalize at the septum. Scale bar: 1 μm. (B) Localization of TagO–GFP and PBP4–mCherry was analyzed in 902 cells in the early stages of septum formation. Localization of each protein was assigned to three sequential stages: scattering around the entire membrane; localization in a ring around the division plane, usually seen as two spots; and localization over the entire closed septum, usually seen as a line across the cell. In 44% of the cells, TagO was found at the septum “ahead” of PBP4, meaning that either TagO is already at the septum, seen as two spots, whereas PBP4 is still scattered around the cell membrane or that TagO is already across the entire septum, whereas PBP4 is still in a ring around the division septum.
Fig. 4.
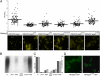
Synthesis of teichoic acids, and not TagO protein itself, is required for PBP4 recruitment to the division septum. (A) Quantification of septum versus lateral membrane fluorescence (fluorescence ratios, FR) and fluorescence microscopy images for PBP4–YFP protein fusion in Δ_tagO_ mutants complemented with wild-type TagO protein (RNΔ_tagO_PBP4YFPp_tagOwt_) or with different TagO mutants (RNΔ_tagO_PBP4YFPp_tagO_D87A, D88A, D87A/D88A, G152A, and N198A). Quantification was performed in 100 cells that displayed closed septa for each strain. Horizontal lines correspond to average FR values. FR values over 2 indicate septal localization, and FR values equal to or under 2 indicate that a protein is dispersed over the cell surface. p values < 10−7. Scale bar: 1 μm. (B) WTAs were isolated from RNΔ_tagO_p_tagO_ and RNΔ_tagO_p_tagO_D87A, D88A, D87A/D88A, G152A, and N198A and analyzed by native PAGE stained with alcian blue/silver stain. Mutations of the aspartic acids and glycine residues led to a decrease or absence of the WTAs. (C) Comparison between the levels of WTA and the degree of PBP4 localization to the division septa (calculated as described in
Materials and Methods
) indicates a strong correlation between the amount of WTA present in the cell and the ability of PBP4 to localize at the septum. (D) Fluorescence microscopy images of RNTagOwtGFP and RNTagOG152AGFP showing that the TagOG152A–GFP fusion localizes to the division septum, similarly to the GFP fusion to the wild-type TagO protein. Scale bar: 1 μm.
Fig. 5.
Model for the role of teichoic acids synthesis in PBP4 recruitment to the septum. The early cell-wall synthetic machinery assembles at the division site, leading to the synthesis of new PGN, with low levels of cross-linking (Left). TagO (together with other WTA synthetic enzymes) is recruited to the septum by an unknown mechanism, leading to the synthesis of intermediate molecules in TA biosynthesis (Center). These intermediates (or another cellular component dependent on TA biosynthesis) function as a temporal and spatial cue for PBP4 recruitment to the division septum, allowing the synthesis of highly cross-linked PGN to occur in a regulated manner (Right).
Similar articles
- Wall teichoic acids of Staphylococcus aureus limit recognition by the drosophila peptidoglycan recognition protein-SA to promote pathogenicity.
Atilano ML, Yates J, Glittenberg M, Filipe SR, Ligoxygakis P. Atilano ML, et al. PLoS Pathog. 2011 Dec;7(12):e1002421. doi: 10.1371/journal.ppat.1002421. Epub 2011 Dec 1. PLoS Pathog. 2011. PMID: 22144903 Free PMC article. - Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus.
Gründling A, Schneewind O. Gründling A, et al. J Bacteriol. 2006 Apr;188(7):2463-72. doi: 10.1128/JB.188.7.2463-2472.2006. J Bacteriol. 2006. PMID: 16547033 Free PMC article. - Inhibitory role for D-alanylation of wall teichoic acid in activation of insect Toll pathway by peptidoglycan of Staphylococcus aureus.
Tabuchi Y, Shiratsuchi A, Kurokawa K, Gong JH, Sekimizu K, Lee BL, Nakanishi Y. Tabuchi Y, et al. J Immunol. 2010 Aug 15;185(4):2424-31. doi: 10.4049/jimmunol.1000625. Epub 2010 Jul 16. J Immunol. 2010. PMID: 20639481 - LytR-CpsA-Psr Glycopolymer Transferases: Essential Bricks in Gram-Positive Bacterial Cell Wall Assembly.
Stefanović C, Hager FF, Schäffer C. Stefanović C, et al. Int J Mol Sci. 2021 Jan 18;22(2):908. doi: 10.3390/ijms22020908. Int J Mol Sci. 2021. PMID: 33477538 Free PMC article. Review. - Function and regulation of Staphylococcus aureus wall teichoic acids and capsular polysaccharides.
Keinhörster D, George SE, Weidenmaier C, Wolz C. Keinhörster D, et al. Int J Med Microbiol. 2019 Sep;309(6):151333. doi: 10.1016/j.ijmm.2019.151333. Epub 2019 Jul 18. Int J Med Microbiol. 2019. PMID: 31362856 Review.
Cited by
- The Staphylococcus aureus cell division protein, DivIC, interacts with the cell wall and controls its biosynthesis.
Tinajero-Trejo M, Carnell O, Kabli AF, Pasquina-Lemonche L, Lafage L, Han A, Hobbs JK, Foster SJ. Tinajero-Trejo M, et al. Commun Biol. 2022 Nov 11;5(1):1228. doi: 10.1038/s42003-022-04161-7. Commun Biol. 2022. PMID: 36369270 Free PMC article. - Chemical Genetic Analysis and Functional Characterization of Staphylococcal Wall Teichoic Acid 2-Epimerases Reveals Unconventional Antibiotic Drug Targets.
Mann PA, Müller A, Wolff KA, Fischmann T, Wang H, Reed P, Hou Y, Li W, Müller CE, Xiao J, Murgolo N, Sher X, Mayhood T, Sheth PR, Mirza A, Labroli M, Xiao L, McCoy M, Gill CJ, Pinho MG, Schneider T, Roemer T. Mann PA, et al. PLoS Pathog. 2016 May 4;12(5):e1005585. doi: 10.1371/journal.ppat.1005585. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27144276 Free PMC article. - Dual role for the O-acetyltransferase OatA in peptidoglycan modification and control of cell septation in Lactobacillus plantarum.
Bernard E, Rolain T, David B, André G, Dupres V, Dufrêne YF, Hallet B, Chapot-Chartier MP, Hols P. Bernard E, et al. PLoS One. 2012;7(10):e47893. doi: 10.1371/journal.pone.0047893. Epub 2012 Oct 26. PLoS One. 2012. PMID: 23110121 Free PMC article. - Dual roles of FmtA in Staphylococcus aureus cell wall biosynthesis and autolysis.
Qamar A, Golemi-Kotra D. Qamar A, et al. Antimicrob Agents Chemother. 2012 Jul;56(7):3797-805. doi: 10.1128/AAC.00187-12. Epub 2012 May 7. Antimicrob Agents Chemother. 2012. PMID: 22564846 Free PMC article. - MreB filaments align along greatest principal membrane curvature to orient cell wall synthesis.
Hussain S, Wivagg CN, Szwedziak P, Wong F, Schaefer K, Izoré T, Renner LD, Holmes MJ, Sun Y, Bisson-Filho AW, Walker S, Amir A, Löwe J, Garner EC. Hussain S, et al. Elife. 2018 Feb 22;7:e32471. doi: 10.7554/eLife.32471. Elife. 2018. PMID: 29469806 Free PMC article.
References
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials