Pericytes are required for blood-brain barrier integrity during embryogenesis - PubMed (original) (raw)
. 2010 Nov 25;468(7323):562-6.
doi: 10.1038/nature09513. Epub 2010 Oct 13.
Affiliations
- PMID: 20944625
- PMCID: PMC3241506
- DOI: 10.1038/nature09513
Pericytes are required for blood-brain barrier integrity during embryogenesis
Richard Daneman et al. Nature. 2010.
Abstract
Vascular endothelial cells in the central nervous system (CNS) form a barrier that restricts the movement of molecules and ions between the blood and the brain. This blood-brain barrier (BBB) is crucial to ensure proper neuronal function and protect the CNS from injury and disease. Transplantation studies have demonstrated that the BBB is not intrinsic to the endothelial cells, but is induced by interactions with the neural cells. Owing to the close spatial relationship between astrocytes and endothelial cells, it has been hypothesized that astrocytes induce this critical barrier postnatally, but the timing of BBB formation has been controversial. Here we demonstrate that the barrier is formed during embryogenesis as endothelial cells invade the CNS and pericytes are recruited to the nascent vessels, over a week before astrocyte generation. Analysing mice with null and hypomorphic alleles of Pdgfrb, which have defects in pericyte generation, we demonstrate that pericytes are necessary for the formation of the BBB, and that absolute pericyte coverage determines relative vascular permeability. We demonstrate that pericytes regulate functional aspects of the BBB, including the formation of tight junctions and vesicle trafficking in CNS endothelial cells. Pericytes do not induce BBB-specific gene expression in CNS endothelial cells, but inhibit the expression of molecules that increase vascular permeability and CNS immune cell infiltration. These data indicate that pericyte-endothelial cell interactions are critical to regulate the BBB during development, and disruption of these interactions may lead to BBB dysfunction and neuroinflammation during CNS injury and disease.
Figures
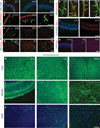
Figure 1. Time course of cell generation and BBB development in the rat cerebral cortex
a–g, Sections of rat cerebral cortex at indicated ages were stained for endothelial cells with Bandeiraea simplicifolia lectin I (BSL) (green, a–f) and nuclei with DAPI (blue, a, f (left), g), pericytes with anti-PDGFR-β (red, b; white arrows point to pericytes), oligodendrocyte progenitors with anti-PDGFR-α (red, c), astrocytes with anti-aquaporin 4 (red, d), anti-occludin (red, e; yellow arrows indicate tight-junction strands), anti-Glut1 (red, f (right)), and anti-Pgp (red, g). Scale bars represent 100 µm (a–d, f, g) and 20 µm (e). h–j, Rats aged E15 (left), E21 (middle) and adults (right) were given a trans-cardiac perfusion of biotin, and liver (h), muscle (i) and brain (j) tissue sections were stained with streptavidin (green) and DAPI (blue). Scale bar represents 100 µm.
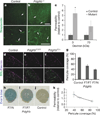
Figure 2. Pericytes are required for BBB formation
a, b, E18 _Pdgfrb_−/− mice (b) and littermate controls (a) were given a trans-cardiac perfusion of biotin, and tissue sections were stained with streptavidin (green; white arrows indicate tracer in vessels). Scale bars represent 200 µm (upper panel) and 100 µm (lower panel). c, E18 _Pdgfrb_−/− mice and littermate controls were given a trans-cardiac perfusion of 3 kDa or 70 kDa biotinylated dextran, tissue sections stained with streptavidin-Alexa 488, fluorescence was quantified in ImageJ and permeability relative to control was graphed. *P < 0.05 by Student’s _t_-test. d–f, Neonatal mouse cerebral cortex from _Pdgfrb_F7/− (f), _Pdgfrb_F7/F7 (e) and littermate controls (d) were stained with BSL (green, d–f (bottom)) and for pericytes with anti-desmin (purple, d–f). Scale bar represents 100 µm. g, Pericyte coverage of CNS vessels in _Pdgfrb_F7/−, _Pdgfrb_F7/F7 and littermate control mice was quantified by analysing per cent length of BSL+ vessels opposed to desmin+ pericytes. h–j, P5 _Pdgfrb_F7− mice (h), _Pdgfrb_F7/F7 mice (i) and littermate controls (j) were given an intraperitoneal injection of Evan’s blue dye, and their brains were dissected the following day after PBS perfusion. k, Neonatal _Pdgfrb_F7/−, _Pdgfrb_F7/F7 and littermate controls were given a trans-cardiac perfusion of biotin and leakage was quantified in tissue sections with streptavidin-Alexa-488 (y axis) and graphed versus pericyte coverage (x axis; values from panel g). All error bars represent s.e.m.
Figure 3. Pericytes regulate structural aspects of the BBB
A, B, Electron microscopy images of CNS vessels from E18 _Pdgfrb_−/− mice (B) and littermate controls (A) including whole endothelial cell cross-sections (a), cytoplasm (b; yellow arrows indicate cytoplasmic vessels), tight junctions (c; yellow arrows indicate altered junction alignment; yellow arrowheads indicate junctions dipping into parenchyma), and after perfusion with biotin followed by staining with streptavidin–HRP (d, e; white arrows indicate uptake of tracer). Scale bars represent 2 µm (a), 0.5 µm (b, c) and 0.2 µm (d, e). C, Quantification of the number of vesicles per endothelial cross-section for _Pdgfrb_−/− mice and littermates. D, Angles of tight junctions (TJs) for _Pdgfrb_−/− mice and littermate controls were classified as parallel to the lumen (0°), perpendicular to the lumen (90°) or in between (45°). *P < 0.05 by Student’s _t_-test. E, F, Cerebral cortex of E18 _Pdgfrb_−/− mice (F) and littermate controls (E) were stained with BSL (green) and anti-occludin (red). Scale bars represent 20 µm. G, H, Purified murine brain endothelial cells were cultured alone (G) or with a feeding layer of purified brain pericytes (H) and stained with DAPI (blue) and anti-claudin 5 (left, red) or anti-occludin (right, red; yellow arrows indicate cell borders). Scale bars represent 100 µm (left) and 50 µm (right). I, Per cent length of sealed claudin 5 and occludin junctions in endothelial cells cultured alone or with pericyte feeder layers. **P < 0.01 by Student’s _t_-test. J, TEER measurements for purified murine brain endothelial cells cultured alone or with a feeding layer of purified brain pericytes. *P < 0.05 by Student’s _t_-test. All error bars represent s.e.m.
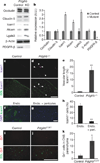
Figure 4. Vascular expression of LAMs in _Pdgfrb_−/− mice
a, b, Western blots of brain lysates from E18 _Pdgfrb_−/− (KO) and littermate controls, probing occludin, claudin 5, Icam1, Alcam, Lgals3, β-actin and PDGFR-β. a, Representative blots; b, quantification; *P < 0.05 by Student’s _t_-test. c–e, Cerebral cortex of E18 _Pdgfrb_−/− mice (d) and littermate controls (c) were stained with anti-Icam1 (purple) and BSL (green, bottom; white arrows indicate Icam1+ vessels), and per cent Icam1+ vascular length was quantified (e). Scale bar represents 250 µm. **P < 0.005 by Student _t_-test. f–h, Purified murine brain endothelial cells cultured alone (f) or with a feeding layer of purified brain pericytes (g) and stained for DAPI (blue) and anti-Icam1 (green), and proportion of Icam1+ cells was quantified (h). Scale bar represents 200 µm. **P < 0.005 by Student _t_-test. i–k, Five-week-old _Pdgfrb_F7/F7 mice (j) and littermate controls (i) were stained with anti-Gr1 (red) and BSL (green, bottom), and number of Gr1+ cells per sagittal section was counted (k). Scale bar represents 250 µm. *P < 0.05 by Students _t_-test. All error bars represent s.e.m.
Comment in
- Blood-brain barrier: Plugging the leak.
Hutchinson E. Hutchinson E. Nat Rev Neurosci. 2010 Dec;11(12):789. doi: 10.1038/nrn2954. Nat Rev Neurosci. 2010. PMID: 21132878 No abstract available.
Similar articles
- Pericytes regulate the blood-brain barrier.
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Armulik A, et al. Nature. 2010 Nov 25;468(7323):557-61. doi: 10.1038/nature09522. Epub 2010 Oct 13. Nature. 2010. PMID: 20944627 - CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development.
Chen J, Luo Y, Hui H, Cai T, Huang H, Yang F, Feng J, Zhang J, Yan X. Chen J, et al. Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7622-E7631. doi: 10.1073/pnas.1710848114. Epub 2017 Aug 21. Proc Natl Acad Sci U S A. 2017. PMID: 28827364 Free PMC article. - Mfsd2a is critical for the formation and function of the blood-brain barrier.
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Ben-Zvi A, et al. Nature. 2014 May 22;509(7501):507-11. doi: 10.1038/nature13324. Epub 2014 May 14. Nature. 2014. PMID: 24828040 Free PMC article. - Targetability of the neurovascular unit in inflammatory diseases of the central nervous system.
Smith BC, Tinkey RA, Shaw BC, Williams JL. Smith BC, et al. Immunol Rev. 2022 Oct;311(1):39-49. doi: 10.1111/imr.13121. Epub 2022 Jul 31. Immunol Rev. 2022. PMID: 35909222 Free PMC article. Review. - Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease.
Hill J, Rom S, Ramirez SH, Persidsky Y. Hill J, et al. J Neuroimmune Pharmacol. 2014 Dec;9(5):591-605. doi: 10.1007/s11481-014-9557-x. Epub 2014 Aug 14. J Neuroimmune Pharmacol. 2014. PMID: 25119834 Free PMC article. Review.
Cited by
- The role of pericytes in hyperemia-induced capillary de-recruitment following stenosis.
Kaul S, Methner C, Mishra A. Kaul S, et al. Curr Tissue Microenviron Rep. 2020 Dec;1(4):163-169. doi: 10.1007/s43152-020-00017-6. Epub 2020 Oct 30. Curr Tissue Microenviron Rep. 2020. PMID: 33778770 Free PMC article. - LRP-1-mediated intracellular antibody delivery to the Central Nervous System.
Tian X, Nyberg S, S Sharp P, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, Battaglia G. Tian X, et al. Sci Rep. 2015 Jul 20;5:11990. doi: 10.1038/srep11990. Sci Rep. 2015. PMID: 26189707 Free PMC article. - Blood-Brain Barrier Disruption and Neurovascular Unit Dysfunction in Diabetic Mice: Protection with the Mitochondrial Carbonic Anhydrase Inhibitor Topiramate.
Salameh TS, Shah GN, Price TO, Hayden MR, Banks WA. Salameh TS, et al. J Pharmacol Exp Ther. 2016 Dec;359(3):452-459. doi: 10.1124/jpet.116.237057. Epub 2016 Oct 11. J Pharmacol Exp Ther. 2016. PMID: 27729477 Free PMC article. - Neuronal Activity Regulates Blood-Brain Barrier Efflux Transport through Endothelial Circadian Genes.
Pulido RS, Munji RN, Chan TC, Quirk CR, Weiner GA, Weger BD, Rossi MJ, Elmsaouri S, Malfavon M, Deng A, Profaci CP, Blanchette M, Qian T, Foreman KL, Shusta EV, Gorman MR, Gachon F, Leutgeb S, Daneman R. Pulido RS, et al. Neuron. 2020 Dec 9;108(5):937-952.e7. doi: 10.1016/j.neuron.2020.09.002. Epub 2020 Sep 25. Neuron. 2020. PMID: 32979312 Free PMC article. - Transgenic animal models to explore and modulate the blood brain and blood retinal barriers of the CNS.
Goncalves A, Antonetti DA. Goncalves A, et al. Fluids Barriers CNS. 2022 Nov 1;19(1):86. doi: 10.1186/s12987-022-00386-0. Fluids Barriers CNS. 2022. PMID: 36320068 Free PMC article. Review.
References
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. - PubMed
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail-chick transplantation chimeras. Dev. Biol. 1981;84:183–192. - PubMed
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. - PubMed
- Bauer HC, et al. Neovascularization and the appearance of morphological characteristics of the blood-brain barrier in the embryonic mouse central nervous system. Brain Res. Dev. Brain Res. 1993;75:269–278. - PubMed
- Bolz S, Farrell CL, Dietz K, Wolburg H. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood-brain barrier in rats. Cell Tissue Res. 1996;284:355–365. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous