Exon junction complex subunits are required to splice Drosophila MAP kinase, a large heterochromatic gene - PubMed (original) (raw)
Exon junction complex subunits are required to splice Drosophila MAP kinase, a large heterochromatic gene
Jean-Yves Roignant et al. Cell. 2010.
Abstract
The exon junction complex (EJC) is assembled on spliced mRNAs upstream of exon-exon junctions and can regulate their subsequent translation, localization, or degradation. We isolated mutations in Drosophila mago nashi (mago), which encodes a core EJC subunit, based on their unexpectedly specific effects on photoreceptor differentiation. Loss of Mago prevents epidermal growth factor receptor signaling, due to a large reduction in MAPK mRNA levels. MAPK expression also requires the EJC subunits Y14 and eIF4AIII and EJC-associated splicing factors. Mago depletion does not affect the transcription or stability of MAPK mRNA but alters its splicing pattern. MAPK expression from an exogenous promoter requires Mago only when the template includes introns. MAPK is the primary functional target of mago in eye development; in cultured cells, Mago knockdown disproportionately affects other large genes located in heterochromatin. These data support a nuclear role for EJC components in splicing a specific subset of introns.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
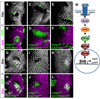
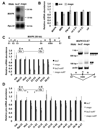
Figure 1. mago is required for EGFR-dependent processes in the eye and wing discs
(A) Photoreceptor differentiation proceeds from posterior (P) to anterior (A) across the eye disc. R8 cells differentiate first, immediately posterior to the MF, and produce the ligand Spi, which activates the EGFR in surrounding cells and induces the sequential formation of R1–R7 cells. (B) Sequence comparison of Drosophila Mago and human Magoh. Identical amino acids are in red. The amino acid changes in our three mago alleles are indicated. (C-N) third instar eye imaginal discs with anterior to the left. Photoreceptors are stained with anti-Elav (C, E, G, blue in D, F, H, J, L). R8 is stained with anti-Senseless (red in D, F, H). (C, D) wildtype. (E, F) large mago93D mutant clones generated in a Minute background are marked by the absence of GFP (green in F). R8 cells start to differentiate normally, but differentiation of other photoreceptors is impaired. (G, H) large mago93D mutant clones are marked by the absence of GFP (green in H) in discs expressing p35 in all cells posterior to the MF. Rescue of apoptosis in mago mutant clones does not restore R1–R7 differentiation. Insets show enlargements of the boxed regions; note the loss of photoreceptors other than R8 in (E-H). (I-N) mago93D clones are marked by the absence of GFP (green in J, L, N) and stained with anti-Caspase 3 (I, red in J), anti-Cyclin B (K, red in L), or anti-Pointed P1 (M, magenta in N). The mutant clones show increased apoptosis and increased Cyclin B expression (insets show enlargements of boxed regions), indicating a failure to arrest in G1, and do not express Pnt-P1. (O-R) wing discs expressing GFP (green in P) or containing mago93D clones marked by the absence of GFP (green in R). argos-lacZ expression revealed by anti-β-galactosidase staining (O, Q, magenta in P, R) is absent in mago mutant clones.
Figure 2. mago acts downstream of Raf but upstream of phospho-MAPK in the eye disc
(A-L) eye discs in which photoreceptors are stained with anti-Elav (A, C, E, G, I, K; magenta in B, D, F, H, J, L). Clones mutant for mago93D and/or expressing UAS transgenes are labeled by GFP expression (green in B, D, F, H, J, L) and indicated by green arrows in A, C, E, G. I, K. Expression of UAS- sSpi (A, B), UAS-EGFRλtop (C, D), UAS-RasV12 (E, F) or UAS-Raf179 (I, J) does not rescue photoreceptor differentiation in mago mutant clones. (G, H) Expression of UAS- Raf179 in wildtype clones leads to excessive photoreceptor differentiation. (K, L) Expression of UAS-RolledSEM induces excessive photoreceptor differentiation in mago93D clones. (M) a simplified diagram of the EGFR signaling pathway. See also Figure S1.
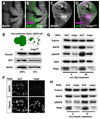
Figure 3. mago is required to maintain normal MAPK protein levels
(A-D) Anti-MAPK staining (A, C, magenta in B, D) of eye discs (A, B) or wing discs (C, D) containing mago93D clones marked by the absence of GFP (green in B, D). MAPK protein levels are strongly reduced in mago mutant clones. (E) Western blot using protein extracts derived either from eye discs expressing GFP in all cells (WT) or from eye discs containing large mago93D clones lacking GFP. The ratio of GFP to Tubulin was used to quantify the amount of remaining wildtype tissue in mago93D mutant eye discs. MAPK levels are decreased by 84% when normalized to GFP. (F) MAPK and Tubulin staining of S2R+ cells treated with lacZ or mago dsRNA. MAPK is specifically reduced. (G) D2F cells treated with lacZ or mago dsRNA were incubated with sSpi conditioned media for 0 or 30 min. Protein lysates were blotted with antibodies to Tubulin, MAPK, diphospho-MAPK, MEK, and Mago. (H) S2R+ cells treated with lacZ or mago dsRNA were incubated with 25 µg/ml insulin for 0 or 10 min. Lysates were blotted with antibodies to Tubulin, MAPK and diphospho-MAPK. mago dsRNA reduced MAPK phosphorylation after sSpi or insulin treatment due to a decrease in total MAPK protein.
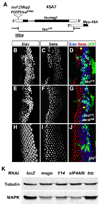
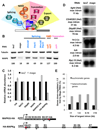
Figure 4. mago, Y14 and eIF4AIII but not btz are required for MAPK expression and function
(A) Genomic structure of Y14, showing the coding regions (black), UTRs (white) and the position of the tsu1 P element and tsu5 allele. The tsuΔ18 deletion removes the whole Y14 open reading frame without disrupting the adjacent gene Mys-45. (B-J) Third instar eye discs containing large clones marked by the absence of GFP (green in D, G, J) homozygous for the Y14 null allele tsuΔ18 (B-D); for tsuΔ18, darkN28 (E-G); and for btz2 (H-J). Photoreceptors are stained with anti-Elav (B, E, H; blue in D, G, J) and R8 is stained with anti-Sens (C, F, I; red in D, G, J). Arrows in D, G point to clusters containing only R8. Like mago, Y14 is required independently for both photoreceptor differentiation and cell survival, but btz is not necessary for either. (K) Protein lysates from S2R+ cells treated with the indicated dsRNAs were blotted with antibodies to Tubulin and MAPK. Mago, Y14 and eIF4AIII are required to maintain MAPK levels, but Btz is not. See also Figure S2.
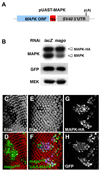
Figure 5. A MAPK cDNA rescues photoreceptor differentiation in mago mutant cells
(A) Diagram of the MAPK-HA cDNA construct. (B) Western blot of protein extracts from S2R+ cells transfected with UAS-MAPK cDNA, UAS-GFP and _actin_-GAL4, and treated with lacZ or mago dsRNA. Expression of endogenous MAPK (lower band), but not the HA-tagged MAPK cDNA (upper band), is reduced in the absence of Mago. (C-H) Eye discs containing mago93D clones alone (C, D) or mago93D clones expressing MAPK cDNA (E-H), positively marked by GFP expression (H, green in D, F) and by anti-HA staining (G, blue in F). Photoreceptors are stained with anti-Elav (C, E; red in D, F). MAPK cDNA restores almost normal photoreceptor differentiation to mago mutant cells. See also Figure S3.
Figure 6. Mago affects MAPK mRNA levels post-transcriptionally
(A) MAPK and Ribosomal protein L32 (RpL32) mRNA detected by Northern blotting in S2R+ cells treated with lacZ or mago dsRNA. (B) Transcript levels in S2R+ cells treated with the control dsRNA aveugle (ave), an upstream component of the EGFR pathway, or with mago dsRNA were measured by qRT-PCR. mago RNAi reduced MAPK mRNA levels by 70%, but did not affect mRNAs encoding other EGFR pathway components. (C) Diagram showing the 7 exons and 6 introns of the 50 kb MAPK gene. Primers spanning each exon-exon junction were used to detect mRNA levels by qRT-PCR in lacZ or mago dsRNA-treated S2R+ cells. (D) MAPK pre-mRNA levels in S2R+ cells treated with lacZ or mago dsRNA were assessed by qRT-PCR using primers spanning the exon-intron junctions. Pre-mRNA levels are reduced in some regions of the MAPK gene and increased in others in Mago-depleted cells, suggesting that Mago does not affect MAPK transcription. For C and D, the mean of 5 experiments is shown; error bars indicate standard deviations. β-tubulin (tub), RpL15 and Histone H3 (His3) were used as controls. Signals detected in the absence of reverse transcriptase are also plotted (_lacZ_-no RT, _mago_-no RT) but are negligible on the scale of these graphs. (E) RT-PCR using primers in exons 3 and 7 amplified a smaller product in cells treated with mago, but not lacZ, dsRNA (arrow). The structure of this product is diagrammed below. See also Figure S4 and Table S2.
Figure 7. The pre-EJC may facilitate splicing of large introns in heterochromatic genes
(A) Proteins associated with the core EJC and their known functions. (B) MAPK and Tubulin levels detected by Western blotting of lysates from S2R+ cells treated with the indicated dsRNAs. Quantification of MAPK levels relative to the lacZ control is shown below the blot. Knocking down the splicing factors SRm160 and RnpS1, especially in combination, reduces MAPK levels. (C) qRT-PCR was used to measure mRNA transcribed from the UAS-HA-MAPK genomic construct shown below (HA-MAPKg, primers in the HA sequence and exon 2) and from actin-MAPKi5-HA (primers in exon 6 and the HA sequence) in S2R+ cells treated with lacZ or mago dsRNA. Controls were tub, RpL15, Dbp80, and endogenous MAPK (primers in exons 2 and 3), and HA-MAPKg levels were normalized to transcripts from cotransfected UAS-GFP, also driven by _actin_-GAL4. Mago depletion reduces mRNA expressed from the genomic construct. (D) Northern blots of RNA from S2R+ cells treated with lacZ or mago dsRNA. Expression of the large heterochromatic (Het) genes light, CG40263 and Dbp80 is reduced by mago RNAi, but expression of the small genes tub, 14-3-3 ε and RpL 15 is unaffected. (E) Genes downregulated ≥1.5 fold in mago dsRNA-treated S2R+ cells broken down by location in euchromatin or heterochromatin and by the size of their largest introns are shown as a percentage of the total number of genes in each category that are expressed in control lacZ dsRNA-treated cells. Heterochromatic genes with introns larger than 15 kb are the most likely to be dependent on Mago. See also Figure S5 and Table S1.
Similar articles
- Exon junction complex proteins bind nascent transcripts independently of pre-mRNA splicing in Drosophila melanogaster.
Choudhury SR, Singh AK, McLeod T, Blanchette M, Jang B, Badenhorst P, Kanhere A, Brogna S. Choudhury SR, et al. Elife. 2016 Nov 23;5:e19881. doi: 10.7554/eLife.19881. Elife. 2016. PMID: 27879206 Free PMC article. - Mago Nashi and Tsunagi/Y14, respectively, regulate Drosophila germline stem cell differentiation and oocyte specification.
Parma DH, Bennett PE Jr, Boswell RE. Parma DH, et al. Dev Biol. 2007 Aug 15;308(2):507-19. doi: 10.1016/j.ydbio.2007.06.007. Epub 2007 Jun 13. Dev Biol. 2007. PMID: 17628520 Free PMC article. - The exon junction complex controls the splicing of MAPK and other long intron-containing transcripts in Drosophila.
Ashton-Beaucage D, Udell CM, Lavoie H, Baril C, Lefrançois M, Chagnon P, Gendron P, Caron-Lizotte O, Bonneil E, Thibault P, Therrien M. Ashton-Beaucage D, et al. Cell. 2010 Oct 15;143(2):251-62. doi: 10.1016/j.cell.2010.09.014. Cell. 2010. PMID: 20946983 - The Physiological Roles of the Exon Junction Complex in Development and Diseases.
Asthana S, Martin H, Rupkey J, Patel S, Yoon J, Keegan A, Mao Y. Asthana S, et al. Cells. 2022 Apr 1;11(7):1192. doi: 10.3390/cells11071192. Cells. 2022. PMID: 35406756 Free PMC article. Review. - Exon Junction Complexes: Supervising the Gene Expression Assembly Line.
Boehm V, Gehring NH. Boehm V, et al. Trends Genet. 2016 Nov;32(11):724-735. doi: 10.1016/j.tig.2016.09.003. Epub 2016 Sep 22. Trends Genet. 2016. PMID: 27667727 Review.
Cited by
- Mouse models of Casc3 reveal developmental functions distinct from other components of the exon junction complex.
Mao H, Brown HE, Silver DL. Mao H, et al. RNA. 2017 Jan;23(1):23-31. doi: 10.1261/rna.058826.116. Epub 2016 Oct 25. RNA. 2017. PMID: 27780844 Free PMC article. - The exon junction complex is required for definition and excision of neighboring introns in Drosophila.
Hayashi R, Handler D, Ish-Horowicz D, Brennecke J. Hayashi R, et al. Genes Dev. 2014 Aug 15;28(16):1772-85. doi: 10.1101/gad.245738.114. Epub 2014 Jul 31. Genes Dev. 2014. PMID: 25081352 Free PMC article. - Multifunctional RNA processing protein SRm160 induces apoptosis and regulates eye and genital development in Drosophila.
Fan YJ, Gittis AH, Juge F, Qiu C, Xu YZ, Rabinow L. Fan YJ, et al. Genetics. 2014 Aug;197(4):1251-65. doi: 10.1534/genetics.114.164434. Epub 2014 Jun 6. Genetics. 2014. PMID: 24907259 Free PMC article. - The exon junction complex is required for DMD gene splicing fidelity and myogenic differentiation.
Da Cunha D, Miro J, Van Goethem C, Notarnicola C, Hugon G, Carnac G, Cossée M, Koenig M, Tuffery-Giraud S. Da Cunha D, et al. Cell Mol Life Sci. 2024 Mar 21;81(1):150. doi: 10.1007/s00018-024-05188-1. Cell Mol Life Sci. 2024. PMID: 38512499 Free PMC article. - Multifaceted Regulation of Gene Expression by the Apoptosis- and Splicing-Associated Protein Complex and Its Components.
Deka B, Singh KK. Deka B, et al. Int J Biol Sci. 2017 Apr 10;13(5):545-560. doi: 10.7150/ijbs.18649. eCollection 2017. Int J Biol Sci. 2017. PMID: 28539829 Free PMC article. Review.
References
- Andersen CB, Ballut L, Johansen JS, Chamieh H, Nielsen KH, Oliveira CL, Pedersen JS, Seraphin B, Le Hir H, Andersen GR. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–1972. - PubMed
- Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861–869. - PubMed
- Bono F, Ebert J, Lorentzen E, Conti E. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell. 2006;126:713–725. - PubMed
- Boswell RE, Prout ME, Steichen JC. Mutations in a newly identified Drosophila melanogaster gene, mago nashi, disrupt germ cell formation and result in the formation of mirror-image symmetrical double abdomen embryos. Development. 1991;113:373–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials